Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Securing Supply Through Onshore Vaccine Manufacturing Capability

Please direct enquiries through our contact page.
Audit snapshot
Why did we do this audit?
- The Australian Government stockpiles a strategic reserve of drugs, vaccines and antidotes to ensure that Australia can remain self-sufficient and meet demand during a national health emergency. In the 2017–18 Federal Budget the Australian Government sought to explore onshore manufacturing options.
- During the COVID-19 pandemic the Australian Government competed with other domestic and international jurisdictions for access to critical medical supplies such as vaccines and personal protective equipment.
Key facts
- In November 2020 the Australian Government signed a 10-year agreement with Seqirus to secure supply of antivenoms, Q fever vaccine and pandemic influenza vaccines through onshore manufacturing from a new facility in Melbourne (owned by Seqirus).
- In March 2022 the Australian Government signed a 10-year agreement with Moderna to secure supply of mRNA vaccines through onshore manufacturing from a new facility in Melbourne (owned by Moderna).
What did we find?
- The procurements achieved the objective of establishing onshore facilities that have the potential to improve supply security. There could have been earlier procurement planning and clearer value-for-money conclusions.
- The Department of Health, Disability and Ageing’s (DHDA) procurement and contract management of the 2020 Seqirus agreement is partly effective.
- DHDA and the Department of Industry, Science and Resources’ (DISR) procurement and contract management of the 2022 Moderna agreement is largely effective.
What did we recommend?
- There were eight recommendations to DHDA, DISR and the Department of Finance related to advice to government; procurement planning; limited tender decision-making; management of probity risks and record keeping; contract management planning; planning for the end of the Seqirus agreement; and protocols for setting aside the Commonwealth Procurement Rules.
- Entities agreed to all recommendations.
1
Number of onshore manufacturers of antivenoms, Q fever vaccine and pandemic influenza vaccines in Australia.
$1.007 billion
Cost to the Australian Government of the Seqirus agreement.
$1.5 to $3 billion
Approximate cost to the Australian Government of the Moderna agreement.
Summary and recommendations
Background
1. In 2020 the Australian Government announced it had signed an agreement with Seqirus (Australia) Pty Ltd (Seqirus, a subsidiary of CSL Limited) whereby Seqirus would invest in the construction of a new, high-tech vaccine manufacturing facility in Melbourne:
to secure Australia’s long-term supply of critical health products including pandemic influenza vaccines and life-saving antivenoms … [and provide] the ability to rapidly manufacture vaccines when responding to health pandemics in the future.1
2. In 2022 the Australian Government announced it had signed an agreement with Moderna Australia Pty Ltd (Moderna):
that secures the production of up to 100 million Australian made mRNA doses every year and hundreds of manufacturing jobs too … which will help protect Australian [sic] against future pandemics while supporting local industry … [and] ensure Australia can meet its ongoing COVID-19 vaccine needs, and any other new and innovative respiratory mRNA vaccines.2
3. The Department of Health, Disability and Ageing (DHDA) is responsible for the 2020 Seqirus procurement and contract management. The Department of Industry, Science and Resources (DISR) was responsible for the approach to market and DISR and DHDA were responsible for the contract negotiations for the 2022 Moderna procurement. DHDA executed the 2022 Moderna contract and is responsible for the contract management of Moderna.
4. DHDA and DISR are non-corporate Commonwealth entities, subject to the Public Governance, Performance and Accountability Act 2013 and prescribed by section 30 of the Public Governance, Performance and Accountability Rules 2014 to comply with the Commonwealth Procurement Rules (CPRs).3
Rationale for undertaking the audit
5. The Australian Government stockpiles a strategic reserve of drugs, vaccines and antidotes to ensure that Australia can remain self-sufficient and meet demand during a national health emergency.4 In the 2017–18 Federal Budget the Australian Government provided funding to support the ongoing production and supply of Q fever vaccine and uniquely Australian antivenoms, and to maintain onshore production of pandemic influenza vaccines. The measure included funding to scope long-term supply options.
6. During the COVID-19 pandemic the Australian Government competed with other domestic and international jurisdictions for access to critical medical supplies such as vaccines and personal protective equipment.
7. The Australian Government has since committed over $4 billion in supporting the establishment of onshore vaccine and other health product manufacturing facilities in Australia.
8. In 2023–24, the Australian Government developed the Buy Australian Plan5 and made the Future Made in Australia Act 20246 to increase domestic manufacturing capability. The audit was identified as a Joint Parliamentary Committee of Public Accounts and Audit priority in 2024–25. The audit provides independent assurance to the Parliament on the procurement and contract management of onshore manufacturing capability for antivenoms, Q fever vaccine, pandemic influenza vaccines and mRNA vaccines for respiratory infections.
Audit objective and criteria
9. The objective of the audit was to examine the effectiveness of the procurement and contract management of onshore manufacturing capability to secure supply of antivenoms and vaccines.
10. To form a conclusion against the objective, the following high-level criteria were applied:
- Was the 2020 procurement of Seqirus onshore manufacturing capability for antivenoms, Q fever vaccine, and influenza vaccines effective?
- Was the 2022 procurement of Moderna onshore manufacturing capability for mRNA vaccines effective?
Conclusion
11. The procurements of onshore manufacturing capability for antivenoms and vaccines have achieved the objective of establishing onshore facilities that have the potential of improving security of supply for Australians. The Seqirus procurement did not maximise value for money for the Australian taxpayer in the long term. For both procurements there could have been earlier procurement planning and justification of a limited tender approach, and a clearer value-for-money conclusion when presenting advice to government.
12. DHDA’s 2020 procurement of onshore manufacturing capability for antivenoms, Q fever vaccine and pandemic influenza vaccines was partly effective. Onshore manufacturing capability to secure supply of critical health products was maintained, however the procurement did not maximise value for money for the Australian taxpayer because:
- the option recommended to government (sole sourcing Seqirus for a further ten years) was not identified in scoping research or legal advice as the option providing the best value for money in the long term;
- advice to government did not comprehensively present the scoping research conclusions;
- there was no approach to market to obtain market intelligence on alternative suppliers;
- conducting the procurement as a sole sourced limited tender was justified under the CPRs after the decision had already been made;
- there was no overall value for money conclusion to support the decision to sole source Seqirus because, in part, there was no comparator; and
- a value for money conclusion made at the end of the contract negotiations with Seqirus was not aligned with the original objective for the procurement, which was to consider novel approaches to ensure long-term sustainable supply.
There was limited procurement planning. The evaluation of Seqirus’ proposals and contract negotiations were consistent with the CPRs within the constraints of the non-competitive option. There were weaknesses in probity planning and processes. There is a contract management plan that was largely implemented as of July 2025, which lacked key elements, including planning for the conclusion of the Seqirus contract in ten years’ time.
13. DHDA’s and DISR’s 2022 procurement of onshore manufacturing capability for mRNA vaccines was largely effective. Onshore manufacturing capability to supply mRNA vaccines to Australians was established at an approximate cost of $1.5 to $3 billion. Research was effectively undertaken to identify mRNA manufacturing options and suppliers. Procurement planning occurred late after a decision to procure Moderna had already been made. Tender evaluation and contract negotiations sought to achieve an economical outcome and were effective. Advice to government was detailed, except that it lacked a clear value for money analysis and conclusion. The procurement was conducted in a manner that was largely consistent with ethical principles. Contract management planning and implementation were largely fit-for-purpose as of July 2025. The contract included provisions for pandemic preparedness. Contract management planning would be enhanced by better planning for the conclusion of the Moderna contract in 2032.
Supporting findings
Seqirus procurement
Table S.1: Summary of Seqirus agreement
|
Agreement clause |
Seqirus contract terms |
|
Date of agreement |
16 November 2020 |
|
Term of agreement |
10 years after the commencement of antivenom and Q fever supplies |
|
Overall cost of agreementa |
$1.007 billion |
|
Initial prepayment included in agreement |
Yes |
|
Ongoing capability payments included in agreement |
Yes |
|
Facility build |
|
|
Requires onshore Australian facility |
Yes, in Melbourne |
|
Ownership of facility |
Seqirus |
|
Facility supply commencement date in agreement |
Yes |
|
Actual date facility is ready to supply |
DHDA advised the ANAO in September 2025 that Seqirus is on track to commence manufacture in the new facility of pandemic influenza vaccines from 1 January 2026 and antivenoms and Q fever vaccines from 30 June 2026 |
|
Ongoing supplies |
|
|
Type of products manufactured |
Antivenoms, Q fever vaccine, and pandemic influenza vaccines |
|
Minimum purchase commitment by government |
No |
|
Can supply other countries |
Yes, provided this does not prevent it from fulfilling its obligations to DHDA |
|
Obligation to prepare for a pandemic |
Seqirus is required to maintain a stockpile of input materials, seed against potential influenza pandemic strains and the required experienced personnel |
Note a: GST inclusive, Australian dollars.
Source: ANAO analysis.
14. A 2015 contract with Seqirus for the supply of Australian antivenoms, Q fever vaccine and pandemic influenza vaccines was due to expire on 30 June 2018. A 2017–18 Federal Budget measure funded scoping of options to ensure long-term supply through sustainable arrangements. Reflecting the long lead times required for market adjustments, between 2016 and 2019 DHDA undertook research that considered pre-existing arrangements, non-procurement alternatives, and costs and benefits of different options, including continuing the arrangement with Seqirus. A 2018 commissioned review stated that a government operator or an open approach to market would provide the greatest economic benefit.
15. In 2019, after an extension of the 2015 Seqirus contract to 2024, DHDA recommended an option to government (sole sourcing of Seqirus) that legal advice, market intelligence and scoping research suggested would not represent the best value for money in the long term. Although there was some analysis of the market’s capacity to competitively respond, DHDA did not approach the market through a formal request for information or other process to obtain market intelligence. Although scoping identified alternative potential suppliers, particularly for pandemic influenza vaccines, the advice justified the recommended option by stating that only Seqirus could deliver the required level of secure onshore supply over the next decade. Advice to government did not comprehensively set out the original objectives or conclusions of the scoping work. Only options involving the extension of the 2015 Seqirus arrangement were recommended to government and pursued.
16. There was a lack of procurement planning (including a clear statement of procurement objectives, a milestone schedule and procurement risk assessment), which could have assisted DHDA to remain focused on the original long-term objectives and to have paced the work effectively. Advice to government and key decisions were not appropriately documented. A decision to conduct the procurement as a sole sourced limited tender was justified under the CPRs after the procurement was already well advanced and decisions had already been made. (See paragraphs 2.3 to 2.35)
17. Although a DHDA business case considered value for money of the Seqirus proposal for ongoing supply, it did not provide an overall value for money conclusion because, in part, there was no alternative provider to test Seqirus’ pricing against. There was no overall value for money conclusion made in tender evaluation. A ‘fair price’ for the goods was provided to government at its request to justify the negotiated value of the contract, however, in part, the ‘fair price’ was based on a Seqirus estimate and not independently verified. Negotiations were informed by due diligence over the Seqirus proposal, and supported by appropriate governance arrangements and expert advice. The value for money conclusion made at the end of the contract negotiations was based on the status quo and not aligned with the original objective for the procurement, which was to consider novel approaches. The value for money conclusion relied in part on research that had been commissioned by Seqirus. The contract was appropriately negotiated and developed, and the expenditure was appropriated authorised and supported by legislation. Procurement outcomes were appropriately reported on AusTender. (See paragraphs 2.36 to 2.50)
18. DHDA did not complete any probity risk assessments. There was no probity plan and a limited conflict of interest declaration process in place prior to July 2020; by this time many decisions had been made, including the decision to sole source Seqirus. In July 2020, as negotiations commenced, DHDA engaged a probity advisor to develop a probity plan and put in place probity arrangements, including a process for declaring potential conflicts of interest. Declaration of interests for the Seqirus procurement was incomplete. A decision against ‘de-bundling’ the different products favoured Seqirus compared to other potential providers. (See paragraphs 2.51 to 2.60)
19. DHDA developed a contract management plan, which lacked key elements, including consideration of ongoing contract risk management and planning for contract transition. As of July 2025 DHDA had largely implemented relevant aspects of its contract management plan. DHDA monitors contract performance through regular reporting from Seqirus. Contract performance management could be improved through consideration of the establishment of performance measures addressing quality, cost, responsiveness/timeliness and/or customer satisfaction. DHDA’s contract with Seqirus has provisions to prepare for an influenza pandemic. (See paragraphs 2.61 to 2.77)
Moderna procurement
Table S.2: Summary of Moderna agreement
|
Agreement clause |
Moderna contract terms |
|
Date of agreement |
Facility establishment agreement signed on 24 March 2022 Non-pandemic supply agreement signed on 12 December 2024 Pandemic advance purchase agreement to be signed in the event of a pandemic if Moderna has a candidate vaccine |
|
Term of agreement |
Until 24 August 2032 |
|
Overall cost of agreementa |
Approximately $1.5 to $3 billionb |
|
Initial prepayment included in agreement |
No |
|
Ongoing capability payments included in agreement |
Yes |
|
Facility build |
|
|
Requires onshore Australian facility |
Yes, in Victoria |
|
Ownership of facility |
Moderna |
|
Facility supply commencement date in agreement |
Yes |
|
Actual date facility is ready to supply |
DHDA advised the ANAO in June 2025 that the facility build is completed and Moderna is seeking final approval from the Therapeutic Goods Administration |
|
Ongoing supplies |
|
|
Type of products manufactured |
mRNA vaccines for respiratory viral infections and other conditionsc |
|
Minimum purchase commitment by government |
Yes, required to purchase a minimum annual dollar value for non-pandemic supply |
|
Can supply other countries |
Yes, provided this does not prevent Moderna from meeting binding orders for pandemic or non-pandemic vaccines placed by the Australian Government |
|
Obligation to prepare for a pandemic |
A pandemic risk management plan outlines operational processes, including how Moderna will obtain input supplies and upscale production if a pandemic is declared |
Note a: GST inclusive, Australian dollars.
Note b: Based on United States to Australian dollar exchange rate 2 July 2025. Range is dependent on a number of factors including the volume purchased.
Note c: As of July 2025, Moderna had two approved mRNA vaccines (COVID-19, Respiratory Syncytial Virus) and 40 in clinical trials.
Source: ANAO analysis.
20. In 2020–21, during the COVID-19 pandemic, DISR and DHDA planned for the ongoing supply of COVID-19 mRNA vaccines. Research included consideration of options, pre-existing arrangements, costs and benefits. In August 2020, DHDA used an open request for information process to obtain market intelligence. In March 2021, DISR and DHDA began negotiating with Moderna to establish an onshore mRNA manufacturing facility for COVID-19 and other vaccines. In May 2021, the government approved finalising negotiations with Moderna and, taking into account the outcomes of negotiations with Moderna, an open approach to market. There was a business plan and draft procurement plan, which defined the goals of the procurement, included some consideration of procurement risk, and estimated the economic benefits. The draft procurement plan was developed after an approach to market had commenced and did not include consideration of any risks related to the unusual parallel procurement process with Moderna. Paragraph 2.6 of the CPRs was used to set the rules aside for the procurement after negotiations with Moderna had commenced. Prior to this time there was no documented consideration of which CPR condition or exemption would apply to justify this limited tender approach. DISR transparently published evaluation criteria for the open approach to market. (See paragraphs 3.5 to 3.32)
21. Fourteen tenders provided a response to the approach to market and were assessed in accordance with a plan and criteria. Moderna did not submit a tender and was included in the assessment as a ‘comparator’ using incomplete and different information to the other proposals. Following the assessment process, which recommended several options, including negotiating with both Moderna and another manufacturer, the government decided to finalise negotiations with both companies. DISR developed a plan and risk assessment for the negotiations and engaged experts. Advice to government was detailed, except that it lacked a clear value for money analysis and conclusion. The options had divergent risks and costs, and the final advice to government was to pursue both options. The government decided to establish an arrangement solely with Moderna at an approximate cost of $1.5 to $3 billion. Following this decision, the Moderna facility establishment agreement and expenditure was appropriately developed, authorised and supported by legislation. In the event of a pandemic, DHDA has the option to order any candidate vaccine developed by Moderna for the relevant infection, after which the parties are required to promptly enter into an advance purchase agreement for pandemic supplies, subject to the negotiation of product price and operational terms. (See paragraphs 3.33 to 3.53)
22. Probity advisers developed probity plans and managed risk. Probity advisors oversaw the management of conflicts of interest. The declaration of interests for the Moderna procurement was incomplete in both DHDA and DISR. DHDA employees declared gifts and benefits appropriately; DISR employees did not. Ethics in procurement includes the equitable treatment of tenderers. There was transparency over the parallel process of conducting an open approach to market while finalising negotiations with one supplier. No tenders were removed from consideration for inconsequential reasons and all tenderers were appropriately informed throughout the process. (See paragraphs 3.56 to 3.70)
23. DHDA has completed contract risk assessments and updates the government on risks and issues. There is a contract management plan, relevant aspects of which DHDA has largely implemented as of July 2025. Contract performance management could have been enhanced through the establishment of specific and direct performance measures that go to quality, cost, responsiveness and/or customer satisfaction. DHDA’s contract with Moderna has provisions to require Moderna to prepare for a pandemic. (see paragraphs 3.71 to 3.83)
Recommendations
Recommendation no. 1
Paragraph 2.18
When advising government on funding options, the Department of Health, Disability and Ageing establish controls to ensure that the advice, if based on research and evidence, is comprehensive and provides an accurate, evidence-based justification for recommending or not recommending options.
Department of Health, Disability and Ageing: Agreed.
Recommendation no. 2
Paragraph 2.26
The Department of Health, Disability and Ageing review and improve controls to ensure that officials undertaking procurements remain focused on the procurement objectives and adhere to an appropriate procurement schedule that enables the achievement of the objectives. This includes the development of procurement plans that clearly set out the objective of the procurement, include procurement risk assessments, and establish appropriate milestones and timeframes.
Department of Health, Disability and Ageing: Agreed.
Recommendation no. 3
Paragraph 2.33
The Department of Health, Disability and Ageing review and improve internal controls on limited tender decision-making, including ensuring that controls support:
- consideration prior to a procurement decision being made about which conditions or exemptions listed in the Commonwealth Procurement Rules justify a limited tender;
- procurement delegates being informed about legal advice; and
- appropriate documentation in record keeping systems of limited tender justifications and related evidence.
Department of Health, Disability and Ageing: Agreed.
Recommendation no. 4
Paragraph 2.58
The Department of Health, Disability and Ageing review and improve controls to ensure:
- probity risks, including potential conflicts of interest, are identified and managed from the early stages of procurements and prior to major procurement decisions being made; and
- appropriate records are kept of relevant meetings with parties to a procurement process, which includes contract management.
Department of Health, Disability and Ageing: Agreed.
Recommendation no. 5
Paragraph 2.65
The Department of Health, Disability and Ageing improve Seqirus contract management planning by: including a fit for purpose risk management plan that is regularly reviewed and incorporating regular review of the contract management plan.
Department of Health, Disability and Ageing: Agreed.
Recommendation no. 6
Paragraph 2.68
The Department for Health, Disability and Ageing commence planning for the conclusion of the Seqirus contract, to ensure sufficient lead time for consideration and (if determined to be value for money) implementation of alternative models for the sustainable long-term supply of antivenoms, Q fever vaccine and pandemic influenza vaccines.
Department of Health, Disability and Ageing: Agreed.
Recommendation no. 7
Paragraph 3.54
The Department of Finance improve guidance to Australian Government entities on:
- the appropriate use of paragraph 2.6 of the Commonwealth Procurement Rules, including how it should be used and revoked for short- and long-term procurements; appropriate timing for its use; and requirements for how its use should be reported on AusTender; and
- how contracts exempt from AusTender reporting should be dealt with under Senate Order 13.
Department of Finance: Agreed.
Recommendation no. 8
Paragraph 3.60
The Department of Industry, Science and Resources implement procurement planning, including probity plans, at the early stages of procurements prior to decisions being made that will determine the direction and influence the outcome of the procurement.
Department of Industry, Science and Resources: Agreed.
Summary of entity response
24. The proposed audit report was provided to DHDA. Extracts of the proposed audit report were provided to DISR, Seqirus, Moderna, the Department of Finance and the Attorney-General’s Department. Summary responses from DHDA and DISR are reproduced below and full responses are provided in Appendix 1. Responses from the Department of Finance and Moderna are provided in Appendix 1. Seqirus and the Attorney-General’s Department did not provide a letter of response. Improvements made by DHDA and DISR observed by the ANAO during the course of this audit are listed at Appendix 2.
Department of Health, Disability and Ageing
The Department of Health, Disability and Ageing (department) welcomes the report’s findings and accepts the recommendations directed to the department. The department is committed to the effective implementation of Australian National Audit Office (ANAO) recommendations and has already taken steps to address the issues identified in this audit.
The department is pleased that the ANAO concluded the Moderna procurement was largely effective. The Seqirus procurement, while only partly effective, was consistent with the Commonwealth Procurement Rules (within the constraints of the non-competitive option) for the evaluation of Seqirus’ proposals and subsequent contract negotiations.
The audit identified areas for improvement in record-keeping, procurement planning, probity and risk management, and the need for clearer value-for-money justifications in advice to government. In response, the department will review and update its internal funding frameworks and guidance to staff developing New Policy Proposals and Ministerial Submissions. This will ensure that funding options presented to government are clearly justified, well evidenced, and properly documented. These actions will reinforce high standards of integrity, accountability, and value for public money in all future procurement activities.
Department of Industry, Science and Resources
The Department of lndustry, Science and Resources (the department) welcomes the ANAO’s finding that the department’s role in securing onshore vaccine manufacturing capability was largely effective. This work occurred at a time when governments across the world were operating at unprecedented speed and scale in uncertain times. The department played an important role in securing the future manufacture of mRNA vaccines in Australia through the Moderna Partnership. The Department acknowledges the importance of early planning, including probity considerations and has implemented several improvements over the past few years. Since 2022, the Department has invested significant time and effort to improve the skills and capabilities of our staff. This includes the establishment of an Integrity Branch, improved guidance and increased support for procurement projects, and improved systems and practices.
Key messages from this audit for all Australian Government entities
25. Below is a summary of key messages, including instances of good practice, which have been identified in this audit and may be relevant for the operations of other Australian Government entities.
Procurement
Contract management
1. Background
Introduction
1.1 The World Health Organization’s (WHO) 2006 Global pandemic influenza action plan to increase vaccine supply sought to increase influenza vaccine production capacity through building new manufacturing facilities in developing and industrialised countries.7 In 2020 the Australian Government announced it had signed an agreement with Seqirus (Australia) Pty Ltd (Seqirus, a subsidiary of CSL Limited) whereby Seqirus would invest $800 million in the construction of a new, high-tech vaccine manufacturing facility in Melbourne.8
1.2 The Australian Government’s coronavirus disease (COVID-19) Response Inquiry9 found that ‘Australia’s lack of onshore manufacturing capability for vaccines other than AstraZeneca left us reliant on international providers and supply chains when issues with this treatment emerged.’10 In many countries, the onshore manufacture of health products, including medicines and vaccines, expanded in response to the COVID-19 pandemic.11 In 2022 the Australian Government announced it had signed an agreement with Moderna Australia Pty Ltd (Moderna) ‘that secures the production of up to 100 million Australian made mRNA doses every year.’12
Seqirus procurement
1.3 Commonwealth Serum Laboratories (CSL) was established as a Commonwealth entity to provide health services to Australians during World War I. CSL has been manufacturing antivenoms since the 1930s, influenza vaccines since the 1940s and Q fever vaccine since 1989 in manufacturing facilities in Melbourne, the United States of America (USA), Switzerland, the United Kingdom (UK) and Germany. In 1993–94 CSL was sold to private investors. In 2012–13 CSL restructured antivenom and Q fever vaccine manufacture into a new subsidiary called bioCSL. bioCSL was renamed Seqirus in 2013.
1.4 In November 2020, the Prime Minister, Minister for Industry, Science and Technology, and the Minister for Health announced an agreement with Seqirus, which was executed after the commencement of the COVID-19 pandemic in early 2020. The announcement stated that:
A new high-tech vaccine manufacturing facility will be developed in Melbourne to secure Australia’s long-term supply of critical health products including pandemic influenza vaccines and life-saving antivenoms. The $1 billion agreement … also provides the ability to rapidly manufacture vaccines when responding to health pandemics in the future.13
1.5 The products referred to in the agreement comprised antivenoms, Q fever vaccine and influenza vaccines.
- Antivenoms — In Australia, on average each year approximately 8,000 people attend hospital with venomous injuries from snakes, spiders and marine creatures, and two to four people die. As of July 2025, 11 creature-specific antivenoms, all manufactured exclusively by Seqirus, were available in Australia.
- Q fever — Q fever is a bacterial infection found worldwide14 and transmitted via aerosols from cattle, sheep and goats. There are approximately 500 cases of Q fever each year in Australia.15 Australia is the only country with a human Q fever vaccine, which is manufactured exclusively by Seqirus.
- Influenza — Influenza is a respiratory tract infection caused by the influenza virus that has caused millions of deaths. There have been four notable influenza pandemics since 1900: the 1918 Spanish flu; the 1957 Asian flu; the 1957 Hong Kong flu; and the 2009 Swine flu.
1.6 The influenza vaccine has been manufactured in eggs for over 70 years. An alternative cell-based technology, available in the USA since 2012, enhances the speed of manufacture, minimises supply chain delays and improves efficacy.16 Seqirus owns egg-based production facilities in Liverpool UK and Melbourne. In 2015 Seqirus acquired Novartis AG’s influenza vaccine business, including a cell-based facility in Holly Springs, North Carolina, USA. The Holly Springs facility can produce 150 million doses of pandemic influenza vaccine within six months of a pandemic being declared, which are controlled by the USA Government.
1.7 In March 2017 the Department of Health, Disability and Ageing (DHDA) advised government that:
- antivenoms and Q fever vaccine manufacture will never be commercially viable and that without ongoing government funding, Seqirus’ production would potentially cease; and
- guaranteed access to pandemic influenza vaccines requires onshore manufacturing capability with government funding ensuring capability and priority access.
1.8 Between 2015 and 2025, the Australian Government provided Seqirus with funding to support its onshore manufacture of antivenoms, Q fever vaccine and pandemic influenza vaccines.
- In 2012–13 CSL sought funding from the Australian Government through a ‘Biosecurity Partnership’ to ensure the ongoing manufacture of antivenoms and Q fever vaccine from its Melbourne facility.
- The 2014–15 Federal Budget Measure ‘Ensuring the supply of antivenoms, Q fever vaccine and Pandemic Influenza vaccines’ provided funding over four years to test the global market for the guaranteed timely supply of Australian antivenoms, Q fever vaccine and pandemic influenza vaccines.
- In 2014–15 Seqirus was the only tenderer in response to a request for tender to supply on the open market antivenoms and Q fever vaccine and maintain onshore manufacturing capability to supply pandemic influenza vaccines. In 2015 the Australian Government signed a three-year contract with Seqirus to secure the onshore manufacture of antivenoms, Q fever vaccine and pandemic influenza vaccines to 30 June 2018. The three-year contract was valued at $140 million.17
- The 2015 contract was extended two times: in 2018 to 30 June 2024; and in 2020 to the point in time that a new manufacturing facility was fully operational (see paragraph 1.9). In total over $545 million will be provided to Seqirus, as reported on AusTender, under the 2015 contract.
1.9 The November 2020 announcement of the agreement with Seqirus18 stated that it required Seqirus to build a new facility in Melbourne19 that is owned by Seqirus, the objective of which is to:
ensure that a timely and assured supply of Supplies is maintained in Australia, in which:
(a) Antivenom Supplies of a range, type and quantity suitable and adequate for the treatment of envenomation peculiar to Australia are available to meet Australian open market demand;
(b) Q Fever Supplies of a type and quantity suitable and adequate for the testing and prevention of Q Fever in Australia are available to meet Australian open market demand; and
(c) Pandemic Supplies of a type and quantity suitable and adequate in the event of a Pandemic, or the threat of a Pandemic, in Australia can be made available for purchase by Health to provide for full Australian population coverage.
1.10 This agreement does not include the supply of pandemic influenza vaccines. The agreement seeks to ensure that Australia has priority rights to pandemic vaccines in the event of an influenza pandemic. The facility is to have the capability to manufacture population scale doses of vaccines in the event of an influenza pandemic. The agreement allows Seqirus to enter into advance purchasing agreements with other countries so long as these will not prevent it from fulfilling its obligations to DHDA. The agreement includes provisions giving the Australian Government a level of priority and certain rights in the event that the supply of Seqirus’ manufacturing facilities cannot meet overall demand for Seqirus’ vaccine supplies.
1.11 As of July 2025 the Melbourne facility build was complete for the manufacture of pandemic influenza vaccines, and Seqirus was in the process of obtaining approvals to commence manufacture. DHDA advised the ANAO in June 2025 of the intention for cell-based influenza vaccine manufacture to commence from 1 January 2026, ahead of the original schedule, and antivenoms and Q fever vaccine from 30 June 2026. Appendix 3 details the timeline of the Seqirus procurement. Most decisions relating to the procurement of Seqirus were made prior to the onset of the COVID-19 pandemic.
Moderna procurement
1.12 Moderna Inc. is an American-owned pharmaceutical company founded in 2010, with manufacturing or research facilities in Australia, Canada, Japan, the UK and the USA.
1.13 COVID-19 is a respiratory tract infection caused by the severe acute respiratory syndrome coronavirus 2 virus. The virus emerged in December 2019 and was declared a pandemic by the WHO in March 2020. As of 5 July 2025 in Australia there had been over 12.2 million cases of COVID-19 and 25,791 deaths from or with COVID-19.20
1.14 The Australian Government’s COVID-19 Response Inquiry noted that ‘in 2020 it was difficult to predict the impact [the pandemic] would have on Australians, or on the health system.’21 There was uncertainty about when or if a vaccine would be developed. By December 2021, COVID-19 vaccines were available in Australia and the Australian Government entered into advance purchasing agreements22 with AstraZeneca PLC (AstraZeneca), the COVID-19 Vaccines Global Access (COVAX) Facility23, Moderna Inc., Novavax Inc. (Novavax), Pfizer Inc. (Pfizer), and the University of Queensland.24
1.15 The three types of COVID-19 vaccines available during the COVID-19 pandemic were25: viral vector, messenger ribonucleic acid (mRNA), and protein subunit.26 AstraZeneca’s COVID-19 vaccine was a viral vector vaccine. The AstraZeneca viral vector vaccine (approved in Australia in February 2021) was the only COVID-19 vaccine manufactured in Australia during the pandemic. Pfizer and Moderna’s COVID-19 vaccines are mRNA vaccines (approved in Australia in January 2021 and August 2021, respectively). Novavax’s COVID-19 vaccine (approved in Australia in January 2022) is a protein subunit vaccine.
1.16 The WHO declared the end of the COVID-19 pandemic in May 2023. By this time, nearly 67 million doses of COVID-19 vaccines had been administered in Australia (Figure 1.1). In June 2021 thrombotic side-effects were associated with the AstraZeneca vaccine and the Australian Technical Advisory Group on Immunisation (ATAGI)27 recommended the Pfizer mRNA vaccine for people aged 16 to 60 years old.28 The Australian Government’s COVID-19 Response Inquiry found that Australia did not have adequate reserves of the Pfizer vaccine onshore, resulting in intermittent supply shortages.29 In total, the Australian Government purchased 28 million doses of Moderna’s COVID-19 mRNA vaccine between May 2021 and July 2024, all of which were manufactured overseas.
Figure 1.1: COVID-19 vaccine doses administered in Australia by brand, February 2021 to May 2023

Note: Data is to 10 May 2023. WHO declared the end of the pandemic on 5 May 2023.
Source: Adapted by ANAO from DHDA, COVID-19 Vaccine Rollout, Canberra, May 2023, available from https://www.health.gov.au/sites/default/files/2023-05/covid-19-vaccine-rollout-update-12-may-2023.pdf [accessed 30 April 2025].
1.17 In 2021 the Australian Government commenced a procurement to: ensure priority access to mRNA vaccines as soon as they are available; provide security of vaccine supply in future pandemics; and strengthen Australia’s biopharmaceutical sector. In March 2022 the Australian Government signed a 10-year ‘facility establishment’ agreement with Moderna to establish an onshore mRNA vaccine manufacturing facility that is owned by Moderna.30 The agreement is in force to August 2032 and comprises: an annual ‘pandemic preparedness facility fee’ paid to Moderna to maintain the mRNA facility; and an annual minimum purchase commitment for mRNA vaccines.
1.18 The facility establishment agreement enables the Australian Government to exercise an option to enter into a pandemic supply agreement provided the Commonwealth orders the vaccines within the contractually-designated window after pandemic declaration.31 Upon exercising this option, the parties must promptly enter into a pandemic supply agreement, which is subject to the negotiation of product price and operational terms. Moderna may supply third parties provided that such supply would not prevent Moderna from meeting binding orders for pandemic or non-pandemic vaccines placed by the Australian Government.
1.19 In December 2024 DHDA signed a ‘non-pandemic supply’ agreement with Moderna, as a sub-agreement to the facility establishment agreement. The non-pandemic supply agreement provides for the purchase of Moderna’s Spikevax COVID-19 mRNA vaccine. Other approved vaccines for respiratory viral infections may be added to the non-pandemic supply agreement.
1.20 The Moderna facility opened in Melbourne on 4 December 2024. DHDA advised the ANAO in June 2025 that the facility is due to begin vaccine output in the second half of 2025, once approval is received from the Therapeutic Goods Administration. Appendix 4 details the timeline of the Moderna procurement.
1.21 As of July 2025 other mRNA manufacturing or research and development facilities were being built in Australia by BioNTech SE (in partnership with Pfizer), the New South Wales Government and BioCina.
Departmental responsibilities for the procurements
1.22 DHDA is responsible for the 2020 Seqirus procurement and contract management. As the Australian Government entity responsible for ‘enabling a productive, resilient and sustainable economy, enriched by science and technology’32, the Department of Industry, Science and Resources (DISR) was responsible for the approach to market and DISR and DHDA were responsible for the contract negotiations for the 2022 Moderna procurement. DHDA executed the 2022 Moderna contract and is responsible for the contract management of Moderna.
1.23 DHDA and DISR are non-corporate Commonwealth entities, subject to the Public Governance, Performance and Accountability Act 2013 and prescribed by section 30 of the Public Governance, Performance and Accountability Rules 2014 to comply with the Commonwealth Procurement Rules.33
Rationale for undertaking the audit
1.24 The Australian Government stockpiles a strategic reserve of drugs, vaccines and antidotes to ensure that Australia can remain self-sufficient and meet demand during a national health emergency.34 In the 2017–18 Federal Budget the Australian Government provided funding to support the ongoing production and supply of Q fever vaccine and uniquely Australian antivenoms, and to maintain onshore production of pandemic influenza vaccines. The measure included funding to scope long-term supply options.
1.25 During the COVID-19 pandemic the Australian Government competed with other domestic and international jurisdictions for access to critical medical supplies such as vaccines and personal protective equipment.
1.26 The Australian Government has since committed over $4 billion in supporting the establishment of onshore vaccine and other health product manufacturing facilities in Australia.
1.27 In 2023–24, the Australian Government developed the Buy Australian Plan35 and made the Future Made in Australia Act 202436 to increase domestic manufacturing capability. The audit was identified as a Joint Parliamentary Committee of Public Accounts and Audit priority in 2024–25. The audit provides independent assurance to the Parliament on the procurement and contract management of onshore manufacturing capability for antivenoms, Q fever vaccine, pandemic influenza vaccines and mRNA vaccines for respiratory infections.
Audit approach
Audit objective, criteria and scope
1.28 The objective of the audit was to examine the effectiveness of the procurement and contract management of onshore manufacturing capability to secure supply of antivenoms and vaccines.
1.29 To form a conclusion against the objective, the following high-level criteria were applied:
- Was the 2020 procurement of Seqirus onshore manufacturing capability for antivenoms, Q fever vaccine, and influenza vaccines effective?
- Was the 2022 procurement of Moderna onshore manufacturing capability for mRNA vaccines effective?
Audit methodology
1.30 The audit methodology included:
- examination of DHDA and DISR documentation;
- review of nine public contributions to the audit from four individuals, three pharmaceutical manufacturing companies, and two peak bodies;
- meetings with officials and staff from DHDA, DISR, Seqirus and Moderna; and
- visits to the new Seqirus and Moderna manufacturing facilities in Melbourne.
1.31 Australian Government entities largely give the ANAO electronic access to records by consent, in a form useful for audit purposes. For the purposes of this audit, DHDA advised the ANAO that it would not voluntarily provide certain information requested by the ANAO due to concerns about its obligations under the Privacy Act 1988, secrecy provisions in DHDA portfolio legislation, confidentiality provisions in contracts and the Public Interest Disclosure Act 2013. To provide comfort to the Secretary regarding DHDA’s obligations under portfolio legislation, on 14 October 2024 the acting Auditor-General issued the Secretary of DHDA with a notice to provide information and produce documents pursuant to section 32 of the Auditor-General Act 1997. Under this notice, DHDA agreed to provide the requested information and documents.
1.32 On 19 September 2025 the acting Secretary of the Department of Health, Disability and Ageing requested, under paragraph 37(1)(a) of the Auditor-General Act 1997 (the Act), that the Auditor-General not include particular information contained within the report because it would unfairly prejudice the commercial interests of bodies mentioned in the report. On 22 October 2025 the Executive Director, Commercial Operations, Seqirus (Australia) Pty Ltd requested, under paragraph 37(1)(a) of the Act, that the Auditor-General not include particular information contained within the report for reasons of unfair commercial prejudice. Paragraph 37(1)(a) of the Act sets out that the Auditor-General must not include particular information in a public report if the Auditor-General is of the opinion that disclosure of the information would be contrary to the public interest for any of the reasons set out in subsection 37(2). Paragraph 37(2)(e) of the Act states that a reason that information should not be disclosed is if it would unfairly prejudice the commercial interests of any body or person. The Auditor-General did not form an opinion that there were public interest grounds under section 37 of the Act to omit the particular information from the report and therefore no information was omitted under section 37. Some editorial changes were however made.
1.33 The audit was conducted in accordance with ANAO Auditing Standards at a cost to the ANAO of approximately $596,000.
1.34 The team members for this audit were Dr Vivian Turner, Mahkaila Sansom, Bezza Wolba, Maggie Lee and Christine Chalmers.
2. Seqirus procurement
Areas examined
This chapter examines whether the Department of Health, Disability and Ageing’s (DHDA) 2020 procurement of manufacturing capability for antivenoms, Q fever vaccine and influenza vaccines was effective.
Conclusion
DHDA’s 2020 procurement of onshore manufacturing capability for antivenoms, Q fever vaccine and pandemic influenza vaccines was partly effective. Onshore manufacturing capability to secure supply of critical health products was maintained, however the procurement did not maximise value for money for the Australian taxpayer because:
- the option recommended to government (sole sourcing Seqirus (Australia) Pty Ltd (Seqirus) for a further ten years) was not identified in scoping research or legal advice as the option providing the best value for money in the long term;
- advice to government did not comprehensively present the scoping research conclusions;
- there was no approach to market to obtain market intelligence on alternative suppliers;
- conducting the procurement as a sole sourced limited tender was justified under the Commonwealth Procurement Rules (CPRs) after the decision had already been made;
- there was no overall value for money conclusion to support the decision to sole source Seqirus because, in part, there was no comparator; and
- a value for money conclusion made at the end of the contract negotiations with Seqirus was not aligned with the original objective for the procurement, which was to consider novel approaches to ensure long-term sustainable supply.
There was limited procurement planning. The evaluation of Seqirus’ proposals and contract negotiations were consistent with the CPRs within the constraints of the non-competitive option. There were weaknesses in probity planning and processes. There is a contract management plan that was largely implemented as of July 2025, which lacked key elements, including planning for the conclusion of the Seqirus contract in ten years’ time.
Areas for improvement
The ANAO made six recommendations to DHDA aimed at providing comprehensive advice to government; improving procurement planning and risk management; documentation of decisions to undertake limited tenders; probity and record keeping in procurements; contract management planning; and planning for the end of the Seqirus contract.
2.1 The CPRs sets out requirements and better practice principles for Australian Government entities undertaking procurement.
- Planning and approach to market — Value for money considerations begin through clearly understanding whether a procurement will deliver best value for money37 and expressing the goals and purpose of the procurement.38 To maximise value for money from a procurement, encouraging competition is a key element of the CPRs.39
- Tender evaluation, contract negotiations and procurement decision-making — Value for money considerations must include financial and non-financial costs and benefits.40
- Probity and ethics — Officials must act ethically throughout a procurement.41
- Record keeping — Documentation commensurate with the scale, scope and risk of the procurement must be maintained.42
2.2 The Australian Government Contract Management Guide (Contract Management Guide) provides the best practice framework for the establishment and management of contracts, which it defines as: ‘all the activities undertaken by an entity, after the contract has been signed or commenced, to manage the performance of the contract (including any corrective action) and to achieve the agreed outcomes.’ 43
Were planning and approach to market consistent with the Commonwealth Procurement Rules?
A 2015 contract with Seqirus for the supply of Australian antivenoms, Q fever vaccine and pandemic influenza vaccines was due to expire on 30 June 2018. A 2017–18 Federal Budget measure funded scoping of options to ensure long-term supply through sustainable arrangements. Reflecting the long lead times required for market adjustments, between 2016 and 2019 DHDA undertook research that considered pre-existing arrangements, non-procurement alternatives, and costs and benefits of different options, including continuing the arrangement with Seqirus. A 2018 commissioned review stated that a government operator or an open approach to market would provide the greatest economic benefit.
In 2019, after an extension of the 2015 Seqirus contract to 2024, DHDA recommended an option to government (sole sourcing of Seqirus) that legal advice, market intelligence and scoping research suggested would not represent the best value for money in the long term. Although there was some analysis of the market’s capacity to competitively respond, DHDA did not approach the market through a formal request for information or other process to obtain market intelligence. Although scoping identified alternative potential suppliers, particularly for pandemic influenza vaccines, the advice justified the recommended option by stating that only Seqirus could deliver the required level of secure onshore supply over the next decade. Advice to government did not comprehensively set out the original objectives or conclusions of the scoping work. Only options involving the extension of the 2015 Seqirus arrangement were recommended to government and pursued.
There was a lack of procurement planning (including a clear statement of procurement objectives, a milestone schedule and procurement risk assessment), which could have assisted DHDA to remain focused on the original long-term objectives and to have paced the work effectively. Advice to government and key decisions were not appropriately documented. A decision to conduct the procurement as a sole sourced limited tender was justified under the CPRs after the procurement was already well advanced and decisions had already been made.
2.3 A 2015 $140 million contract with Seqirus (a subsidiary of CSL Limited) for the supply of Australian antivenoms, Q fever vaccine and pandemic influenza vaccines was due to expire on 30 June 2018 (see paragraph 1.8). DHDA’s 2015 contract with Seqirus was extended twice (in 2018 to 30 June 2024 and in 2020 to the point in time that a new manufacturing facility was fully operational) at a total cost of $545 million. A 2014–15 Federal Budget measure provided funding to DHDA to test the global market for the guaranteed timely supply of these products to ensure efficiency in communicable disease prevention and control.
Procurement planning
Consideration of pre-existing arrangements, non-procurement alternatives and the market’s capacity to competitively respond
2.4 The CPRs state that when a business requirement arises, officials should consider whether a procurement will deliver the best value for money, taking into account factors such as stakeholder input, obligations and opportunities under other existing arrangements.44 In August 2016, internal advice was sought regarding a potential extension of the 2015 Seqirus arrangement beyond 30 June 2018 (the original expiry date). Five options were put forward for further scoping (Table 2.1), which included procurement and non-procurement alternatives. The option of establishing a new Commonwealth entity to manufacture supplies was described as likely to be the most sustainable and cost-effective in the long term.
Table 2.1: Options for the supply of antivenoms, Q fever vaccine and pandemic influenza vaccines, August 2016
|
Option |
Reasoning behind advice |
|
Recommended for further scoping |
|
|
Reform Seqirus arrangement (status quo) |
Continue arrangement with Seqirus but seek to implement changes to increase the long-term sustainability and satisfaction with the arrangement including exploring increased governance, transparency and reporting. |
|
Engage new onshore and offshore suppliers |
Needs to be carefully scoped so that control of the supply arrangement stays with the Commonwealth and cannot be compromised by commercial factors. Requires significant funds to establish capability and meet regulatory requirements. May be made faster if Seqirus agreed to sell some of the technology to a new provider. |
|
New public-private partnership |
Could take many forms and result in shared risk and costs. |
|
Establish a new Commonwealth entity to manufacture supplies |
Likely to be the most sustainable and cost-effective long-term. |
|
Hybrid option for each supply requirement (unbundling of products) |
Could identify the best supply option for each of the three products (antivenoms, Q fever vaccine and pandemic influenza vaccines). |
|
Not recommended for further scoping |
|
|
Doing nothing and allow the market to respond to demand |
Not feasible given the importance of the products. |
|
Engage a vendor to manage the supply chain |
Not recommended as it would be an add on to other options, would need new suppliers with a vendor sitting over the top and would not represent value for money. |
|
DHDA to manage the supply chain |
Not consistent with the role of DHDA and would set a precedent for engaging in commercial activities for high-risk supply requirements. |
|
Acquisition of Seqirus manufacturing sites |
Not recommended given the outdated technology at the sites and the high value of the land. |
|
A joint venture with Seqirus |
Not recommended given the size of Seqirus following the acquisition of Novartis AG’s (see paragraph 1.6), although might have been a viable option with bioCSL. |
Source: Adapted by ANAO from DHDA documentation.
2.5 DHDA received funding through the 2017–18 Federal Budget measure ‘Improving access to medicines — antivenoms, Q fever and pandemic influenza vaccine supply’ to: extend DHDA’s 2015 contract with Seqirus for six years while scoping alternative models of supply. DHDA planned to scope the five policy options described in Table 2.1 from July 2017 to August 2018 and was due to report back to government in the 2019–20 Federal Budget cycle.
2.6 In December 2017 KPMG produced a report evaluating pharmaceutical supply arrangements for pandemic influenza vaccine, Q fever vaccine and uniquely Australian antivenoms (KPMG review), which had been commissioned by DHDA in July 2014.45 The KPMG review examined whether a procurement would deliver best value for money, taking into account a range of considerations: the pre-existing arrangements with Seqirus including its ‘successful features’ and ‘challenges’; and a health economic analysis which compared the costs of the Seqirus arrangement to ‘a more efficient program or another use of the funds invested.’ The KPMG review identified procurement and non-procurement options comprising: a public private partnership; a joint venture; a new Commonwealth entity; prime vendor arrangements; co-funding; and purchaser-provider relationships (similar to the 2015 Seqirus supply option). The December 2017 report concluded that:
Arrangements with Seqirus are effective and appropriate, however they come at an economic loss to the Commonwealth … the outcome for the Australian population’s health would have been better had the Australian Government invested in an alternative, more cost effective opportunity … In practical terms, however, the current purchaser-provider arrangements can be considered to be the only viable approach option to the Commonwealth at this time … evidence suggests that without considerable initial investment from the Commonwealth, an alternative model could not be realised.
2.7 Four further scoping reviews were undertaken in 2018 and 2019 that involved some engagement with other Australian Government entities, technical experts, academics, industry bodies and manufacturers. The four reviews considered the existing arrangement with Seqirus, other procurement options and non-procurement alternatives (Appendix 5).46
- May 2018 — Certara, Therapeutic needs and opportunities assessment.47
- August 2018 — PharmOut, Biopharmaceutical manufacturing facility study.48
- September 2018 — Biointelect and the University of Melbourne, Evidence base to inform policy development regarding future long-term supply arrangements for pandemic influenza vaccine.49
- December 2018 — Ernst and Young (EY), Supply options and business advisory project50 (EY review), updated July 2019.
2.8 The EY review considered risks and benefits, regulatory context, contractual arrangements and modelling and concluded that: no industry organisations were interested in a new public-private partnership with the Australian Government to manufacture all three products; the existing supply model for antivenoms and Q fever vaccine was unsustainable; the bundling together of the different products, while ensuring continued production by Seqirus, was creating a perceived barrier to entry among other suppliers; through its status quo approach, the government did not leverage market intelligence regarding other potential supply solutions; and while the market for Australian antivenoms and Q fever vaccine was limited, there was competition in the provision of influenza vaccines offshore. It listed 14 options, of which five were ‘viable’.
- Option 1 (offshore status quo) — Reform the arrangement with Seqirus using combined onshore and offshore production for the three products, with new technology and onshore facilities.
- Option 2 (onshore status quo) — Reform the arrangement with Seqirus using onshore production for the three products, with new technology and onshore facilities.
- Option 3 (government operator) — Re-establish a Commonwealth entity or task an existing onshore entity (e.g. Commonwealth Scientific and Industrial Research Organisation (CSIRO)).
- Option 4 (onshore market solution) — Separate contracted supply per product category, onshore using new technologies.
- Option 5 (hybrid market solution) — Separate contracted supply per product category, onshore using new technologies, except for antivenoms (which would be supplied offshore).
2.9 The EY review concluded that:
- options 3 (government operator) and 5 (hybrid market solution) had the potential for greatest net benefits;
- while continuity of supply between 2024 and 2028 depended on Seqirus (that is one of the ‘status quo’ options 1 and 2), options 3, 4, and 5 were possible from 2028 onwards; and
- options 3 and 4 had the potential to generate the most favourable outcomes, with option 4 having the benefit of:
[facilitating] new entrants into the Australian market by removing a perceived barrier to entry created by bundling the three product categories. This barrier has been seen to provide preference to the incumbent sole supplier in the tender process based on the feedback from the industry … A direct sourcing arrangement [with Seqirus] as an interim measure while [sic] an open and transparent sourcing process is likely to stimulate considerable interest in the market and produce better outcomes for the government in the longer term.
2.10 The CPRs state that when a business requirement arises, officials should consider whether a procurement will deliver the best value for money, taking into account factors such as the market’s capacity to competitively respond to a procurement.51 The EY review recommended evaluating whether CSIRO or a new government entity could develop new technology for antivenoms and pandemic influenza vaccines; unbundling the product lines; and a stepped procurement through an open request for information, selective or open request for tender (in January–February 2019) and competitive negotiation process. The stepped approach to facilitate the gathering of market intelligence is described in the CPRs as a multi-stage procurement.52 EY noted that this could be conducted in parallel to negotiating an extension of existing supply from Seqirus in the short term.
2.11 In August 2019 DHDA collated information from the 2017, 2018 and 2019 reviews and listed five options, which were mapped back to the options identified for further scoping in August 2016 (see Table 2.1) and were largely aligned with the five EY options.
Figure 2.1: DHDA analysis of viable supply options, August 2019

Source: ANAO based on DHDA documentation.
2.12 Options A and B involved Seqirus establishing onshore facilities, with the main difference between the two options being whether or not the arrangement involved the Australian Government making an upfront capital investment. While DHDA’s analysis favoured option B (upfront capital investment in Seqirus for onshore production of all products) on the basis that it was the ‘most cost effective option in the short term’ and ‘the only option to guarantee continuity of supply’, the analysis also stated that option B ‘may not represent appropriate value for money to the Commonwealth.’ It was ‘highly’ recommended that if option B was selected there should be more robust financial and performance management of Seqirus.
2.13 Option C (new Commonwealth entity) was not recommended due to the high risks associated with government manufacturing pharmaceutical products and DHDA anticipated that establishing a new Commonwealth entity would take six to 10 years. In July and October 2018 CSIRO had provided to DHDA two discussion papers on producing antivenoms and a strategy to improve Q fever vaccine production and diagnostics. DHDA advised the ANAO in February 2025 that it was unable to explain why discussions with CSIRO did not progress.
2.14 In relation to options D and E (debundling the product lines and exploring arrangements with other potential suppliers for one or more of the products), DHDA’s analysis listed potential suppliers for antivenoms, Q fever vaccine, and pandemic influenza vaccines; stated a competitive response might be possible after 2034; and noted that industry consultations identified interest in private-public partnerships and joint ventures. DHDA received proposals from Seqirus during the scoping stage (see paragraphs 2.37 and 2.37), but did not otherwise approach the market to assess the market’s capacity to competitively respond as part of the 2020 procurement. Option E (debundling with some offshore supply arrangements) was not recommended unless adopted as a further risk mitigation in addition to onshore production.
2.15 DHDA’s analysis did not conclude on which option would provide the greatest value for money.
2.16 In November 2019 DHDA provided the Minister for Health and Aged Care (the Minister) a brief on funding options, which stated that a Commonwealth facility carried very high investment, schedule and safety risk; and that options scoping ‘clearly’ showed that only Seqirus could deliver the required level of secure onshore supply over the next decade. The ideas of unbundling the products, seeking proposals from other potential market suppliers for one or more products, directly negotiating with Seqirus for short-term supply while undertaking an approach to market for longer-term supply, or conducting a multi-stage procurement, were not presented to the Minister. Internal planning documentation referred to the possibility of seeking research and development (R&D) funding (including potentially to CSIRO) to stimulate and identify viable market alternatives for antivenoms and Q fever vaccine supply before the end of the next Seqirus arrangement, and to help reduce Seqirus’ ‘monopoly leverage’ in the long term. R&D funding to stimulate the market and increase competition was referred to in the advice to the Minister. The November 2019 advice stated R&D for new generation antivenoms and Q fever vaccine would help ensure greater competition and reduction of the ‘monopoly leverage’ of Seqirus in the longer term.
2.17 In November 2019 the government agreed to the Minister providing advice on a long-term contract with Seqirus, including options A or B (which involved an upfront loan to Seqirus in 2020 and then annual funding) to be considered in the context of the 2020–21 Federal Budget. The approval did not include consideration of new R&D funding.
Recommendation no.1
2.18 When advising government on funding options, the Department of Health, Disability and Ageing establish controls to ensure that the advice, if based on research and evidence, is comprehensive and provides an accurate, evidence-based justification for recommending or not recommending options.
Department of Health, Disability and Ageing response: Agreed.
2.19 The Department of Health, Disability and Ageing will update its internal funding frameworks and guidance documentation to ensure advice to Government about funding options is clearly explained and justified. These changes will recognise the various structured mechanisms through which public funds can be allocated to achieve policy objectives.
Specifying procurement goals, risk, value and benefits
2.20 Resource Management Guide (RMG) 411 Grants, Procurement and other financial arrangements assists non-corporate Commonwealth entities to identify a grant, procurement or other financial arrangement.53 RMG 411 states that the arrangement is more likely to be a grant if it involves financial assistance to build capacity. The 2020 Seqirus financial arrangement had characteristics of a grant.54 Entities are required to document the reason for using a particular financial arrangement and apply the relevant framework. DHDA did not document a rationale for treating the financial arrangement as a procurement.
2.21 DHDA internal guidance dated 2019 states that a procurement plan should be prepared for procurements estimated at $10,000 or more. No procurement plan was developed during the planning stage for the 2020 Seqirus procurement.
2.22 The CPRs state that a thorough consideration of value for money begins by officials ‘clearly understanding and expressing the goals and purpose of the procurement.’55 There was no procurement plan or other authoritative document clearly stating the goals of the procurement. It can be derived from DHDA’s terms of reference for scoping activities funded under the 2017–18 Federal Budget measure (see paragraph 2.5) that the goal was to ensure the long-term supply of antivenoms, Q fever vaccine, and pandemic influenza vaccines through sustainable arrangements that fit with government and public health requirements.
2.23 The Commonwealth Risk Management Policy and CPRs require risk to be considered when making decisions.56 DHDA guidance requires staff to assess risk as early as possible when planning a procurement. Although the scoping work broadly considered risk related to the policy requirement and the different options, no structured risk assessment was developed for the Seqirus 2015 contract extension or 2020 procurement.
2.24 The CPRs state that the expected value of a procurement must be estimated before a decision on the procurement method is made.57 The intent of this requirement is to ensure that the chosen procurement method is consistent with the CPRs and represents value for money. The EY review estimated the total cost of each of the shortlisted options (see Appendix 5). The options of ensuring supply through Seqirus (options 1 and 2) had the highest cost estimates and the option of a government operator (option 3) had the lowest. The November 2019 advice to government did not specify costs.
2.25 The 2020 CPRs required entities to consider economic benefits to the Australian economy for procurements over $4 million.58 The Biointelect study and EY review considered the economic benefit of onshore and offshore supply options (see Appendix 5). Biointelect determined onshore manufacturing to be cost saving in most scenarios through reductions in health care utilisation and preventable deaths. EY identified that continued supply by Seqirus (options 1 and 2) had the lowest gross domestic product increase whilst a new Commonwealth entity (option 3) had the highest.
Recommendation no.2
2.26 The Department of Health, Disability and Ageing review and improve controls to ensure that officials undertaking procurements remain focused on the procurement objectives and adhere to an appropriate procurement schedule that enables the achievement of the objectives. This includes the development of procurement plans that clearly set out the objective of the procurement, include procurement risk assessments, and establish appropriate milestones and timeframes.
Department of Health, Disability and Ageing response: Agreed.
2.27 The Department of Health, Disability and Ageing remains committed to strengthening its procurement guidance and procedural documentation. This includes updating the procurement plan template, to ensure officials use procurement risk assessments and remain focused on the procurement objectives.
Approach to market
Justification of limited tender
2.28 The CPRs and DHDA’s procurement guidance state that for procurements at or above the relevant procurement threshold, limited tender can only be conducted in accordance with conditions listed in paragraph 10.3 or exemptions listed in Appendix A of the CPRs.59 The relevant procurement threshold is $80,000 for non-construction contracts.60
2.29 DHDA’s analysis (see paragraph 2.11) did not explicitly refer to which CPR exemption to open tender would be justified or applicable. DHDA did not provide advice to government on which limited tender condition would apply when recommending the sole sourcing of Seqirus.
2.30 In July 2017 DHDA engaged MinterEllison to provide legal advice61 on the options for future supply of the products following the conclusion of EY’s work. The December 2018 advice stated that there was no major legal impediment to any of the five EY options, subject to DHDA complying with legal and regulatory requirements, including the open tender requirements of the CPRs. The advice stated that:
- divisions 1 and 2 of the CPRs would apply to the procurement as the estimated value was above the relevant threshold;
- the most relevant potential exemptions were set out in paragraphs 10.3.d.ii. and 10.3.d.iii62 of the CPRs, however neither of those tests would be satisfied as the draft EY reports had noted the existence of other entities globally that have access to the relevant technology, know-how, intellectual property (IP) and proprietary rights to produce equivalent products;
- directly engaging with Seqirus was not permitted under the CPRs; and
- if DHDA pursued any procurement option except re-establishing a Commonwealth entity as government operator, it was likely that every time the arrangements expired and it undertook a new procurement, DHDA would find itself in a position where the service provider was able to exert heightened commercial negotiating pressure to maximise its commercial position.
2.31 In August 2020, to inform a brief in which the DHDA Secretary was to be asked to execute a heads of agreement63 with Seqirus, the Australian Government Solicitor (AGS), which had been engaged to provide probity advice (see paragraphs 2.42 and 2.51), sought to understand the background to the decision to sole source Seqirus. DHDA was unable to identify documentation of the decision to proceed with a limited tender. Correspondence between the commercial legal services area, other DHDA officials, AGS and MinterEllison indicates that DHDA was relying on individual officials with ‘corporate memory’ to understand the decision. AGS advised that the information supported DHDA conducting the process as a limited tender under CPR paragraph 10.3(d)(iii) and that this decision needed to be documented, with detail from the ‘market engagement work’ and 2014 tender process. In August 2020 the Secretary for DHDA was informed that negotiations with Seqirus were being progressed as a limited tender under CPR paragraph 10.3.d.iii (due to an absence of competition for technical reasons). The technical reasons were not explained except for a statement that Seqirus was the only manufacturer of antivenoms and Q fever vaccine globally and the only organisation with capability to manufacture pandemic influenza vaccines in Australia.
2.32 For each contract awarded through limited tender, the CPRs require entities to prepare and file in their records management system a written report that includes the value and type of goods and services procured, a statement indicating the circumstances and conditions used to justify the use of limited tender, and a record of how value for money was achieved in the circumstances.64 No report was prepared and filed. In November 2020, following contract negotiations with Seqirus, a minute to the Secretary included these elements.
Recommendation no.3
2.33 The Department of Health, Disability and Ageing review and improve internal controls on limited tender decision-making, including ensuring that controls support:
- consideration prior to a procurement decision being made about which conditions or exemptions listed in the Commonwealth Procurement Rules justify a limited tender;
- procurement delegates being informed about legal advice; and
- appropriate documentation in record keeping systems of limited tender justifications and related evidence.
Department of Health, Disability and Ageing response: Agreed.
2.34 The Department of Health, Disability and Ageing will review and update internal procurement guidance and procedural documentation to improve limited tender decision making and record keeping in line with the Commonwealth Procurement Rules.
Evaluation plan and risk assessment
2.35 The CPRs state that entities should include relevant evaluation criteria in request documentation to enable the proper identification, assessment and comparison of submissions on a fair, common and appropriately transparent basis.65 The Commonwealth Risk Management Policy, CPRs and DHDA internal guidance all emphasise the importance of engaging with risk in a procurement.66 No tender evaluation criteria, evaluation plan or related risk assessment were developed for the 2020 Seqirus procurement.
Were tender evaluation and contract negotiations consistent with the Commonwealth Procurement Rules?
Although a DHDA business case considered value for money of the Seqirus proposal for ongoing supply, it did not provide an overall value for money conclusion because, in part, there was no alternative provider to test Seqirus’ pricing against. There was no overall value for money conclusion made in tender evaluation. A ‘fair price’ for the goods was provided to government at its request to justify the negotiated value of the contract, however, in part, the ‘fair price’ was based on a Seqirus estimate and not independently verified. Negotiations were informed by due diligence over the Seqirus proposal, and supported by appropriate governance arrangements and expert advice. The value for money conclusion made at the end of the contract negotiations was based on the status quo and not aligned with the original objective for the procurement, which was to consider novel approaches. The value for money conclusion relied in part on research that had been commissioned by Seqirus. The contract was appropriately negotiated and developed, and the expenditure was appropriated authorised and supported by legislation. Procurement outcomes were appropriately reported on AusTender.
2.36 The CPRs state that value for money considerations in a procurement must include financial and non-financial costs and benefits including quality of the goods and services, fitness for purpose of the proposal, experience and performance history, and whole-of-life costs.67
Achievement of value for money through tender evaluation
2.37 In September 2018 (as part of the scoping research described in paragraph 2.4) Seqirus provided a ‘preliminary’ proposal to DHDA for post-30 June 2024 supply of antivenoms, Q fever vaccine and pandemic influenza vaccines, with options involving different combinations of onshore, offshore, egg-based and cell-based facilities. Seqirus preferred an option involving a new onshore cell-based facility (described in the proposal as ‘Option C’). Internal advice was that the proposal was ‘high level’ and that value for money could not be accurately assessed for each option. In May 2019 and February 2020 Seqirus submitted new proposals which stated that by 2024 the egg-based facility (see paragraph 1.6) would be obsolete and decommissioned and again proposed onshore production of the three product lines in a new cell-based facility with Australian Government support. Internal documentation from April 2020 stated that an option of a loan was no longer being considered.
2.38 Although there was no tender evaluation plan or criteria (see paragraph 2.35), in May 2020 EY provided DHDA with a Business case for alternative vaccines an antivenom supply arrangements (business case)68, which evaluated the Seqirus proposal. The evaluation used information from a Deloitte Financial Advisory Pty Ltd (Deloitte) review that had been commissioned by Seqirus and considered elements required under the CPRs, including whole of life costs. DHDA’s analysis highly recommended that if the arrangement with Seqirus was continued there should be more robust financial and performance management of Seqirus (see paragraph 2.12). The business case did not specifically consider Seqirus’ performance history. The business case did not provide an overall value for money conclusion and noted that:
The value for money of the Proposed Arrangement can only be considered qualitatively. This is because the bundle of goods being procured is highly bespoke and of national significance, and there is no alternative supplier to easily test pricing for two of the three products.
2.39 The business case included a financial viability assessment of CSL, including Seqirus, based on publicly available information and did not identify any matters of material concern. A steering committee subsequently formed to support negotiations with Seqirus (see paragraph 2.42) noted that ‘the financial information provided by Seqirus is not satisfactory for the Commonwealth to undertake the required due diligence for a proposal of this scale.’69 The Department of Finance (which was a member of the steering committee) sought commercial information from Seqirus to undertake due diligence and Seqirus again provided limited information. Records are unclear as to how this was resolved.
2.40 In advice to government in May 2020, DHDA shared the business case, an assessment of the business case by the Infrastructure and Project Financing Agency70 and three pricing options. DHDA sought a decision by the end of June 2020. Seqirus stated that it required a completed heads of agreement by 31 July 2020. The Minister agreed, subject to DHDA providing a comparison of Seqirus’ proposal against a ‘fair price’ and the cost of the 2015 Seqirus agreement. DHDA calculated and provided to the Minister a ‘fair price’ for the three products, with the ‘fair price’ for antivenoms and Q fever vaccine based on a Seqirus estimate and for pandemic influenza vaccines based on EY analysis of what Australia would pay for an advance purchase agreement on the open market. The ‘fair prices’ closely aligned with the final contract price (see paragraph 2.48).
2.41 In July 2020 the government approved the commencement of formal contract negotiations with Seqirus.
Achievement of value for money through contract negotiations
2.42 The CPRs require entities to consider risks and their potential impact when making decisions relating to value for money assessments, approvals of proposals to spend relevant money and the terms of the contract.71 In August 2020 AGS finalised a ‘governance and process plan’ for DHDA’s contract negotiations with Seqirus.72 The governance and process plan outlined: the governance structure and responsibilities (including a steering committee73 and working group74); a negotiation team comprised of a deputy secretary from DHDA and the Department of Finance; probity arrangements (see paragraph 2.51); and planned negotiation terms.
2.43 The steering committee and working group met or considered updates on multiple occasions between July 2020 and November 2020.
2.44 The steering committee and working group were supported by expert advisors. EY acted as commercial advisor during the negotiations75 and, along with the Department of Finance, aided DHDA in assessing value for money, including through analysis of pricing in October 2020 and November 2020. In July 2020 DHDA engaged King & Wood Mallesons (KWM) as commercial legal advisers.76 KWM worked with DHDA and Seqirus to finalise the contract terms.
2.45 The original intent of the procurement was to ensure the long-term supply of antivenoms, Q fever vaccine, and pandemic influenza vaccines through sustainable arrangements that fit with government and public health requirements and potentially including novel approaches to supply (see paragraph 2.22). In August 2020 the Secretary for DHDA (DHDA Secretary) was advised that value for money was demonstrated through Seqirus being ‘currently the only manufacturer of the relevant antivenoms and Q fever vaccine globally’ and ‘the only organisation with the current capability to manufacture pandemic influenza vaccine in Australia’. The value for money justification was based on the status quo and was not aligned to the procurement objective, which was to seek sustainable and potentially novel solutions, which scoping research had identified as feasible in the long-term.
2.46 On 10 August 2020 the Prime Minister was advised that an in-principle agreement had been reached with Seqirus. A heads of agreement was executed by DHDA and Seqirus on 10 August 2020 and a contract valued at $1.015 billion was executed in November 2020. The contract comprised an Australian Government ‘initial prepayment’ to Seqirus and an annual ‘capability payment’ to commence once the facility was generating supply. As of July 2025, the ten-year agreement was expected to end in June 2036.
2.47 In November 2020 a minute to the DHDA Secretary detailed alignment of the contract with the heads of agreement and value for money. The value for money assessment summarised EY commentary and referenced the Deloitte analysis that had been commissioned by Seqirus. At the end of its engagement as commercial advisor EY stated that:
the agreement achieves continuous onshore supply of the Services and the Commonwealth’s process appears to have maximised the Commonwealth’s advantage in achieving transparency and value in comparison to Seqirus’ original offer.
2.48 The final 10-year agreement was costed at $1.007 billion.
2.49 The expenditure was appropriately authorised by government and supported by legislation; the contract was assessed to be consistent with the heads of agreement; legal and contractual risks were assessed (see paragraph 2.63); and contract indemnity was aligned with Australian Government guidance. The December 2017 KPMG review of DHDA’s 2015 contract with Seqirus (see paragraph 2.6) detailed four opportunities for improvement: increase transparency and reporting; cost-effectiveness (understanding the fixed and variable costs of each product); the quality and efficacy of supplies; and long-term investment in research and development. DHDA’s 2020 contract with Seqirus introduced formal bi-annual progress meetings for the first three years of the contract but removed a requirement for Seqirus to provide financial reporting on how the capability payment was to be used.
AusTender reporting
2.50 The CPRs require entities to report on AusTender contracts within 42 days of signing a contract and report the limited tender condition used (if relevant).77 On 10 December 2020 the contract notice for DHDA’s contract with Seqirus was published on AusTender, within the 42-day requirement. The contract notice stated that the limited tender condition 10.3.d.iii was used.
Were procurement activities conducted ethically?
DHDA did not complete any probity risk assessments. There was no probity plan and a limited conflict of interest declaration process in place prior to July 2020; by this time many decisions had been made, including the decision to sole source Seqirus. In July 2020, as negotiations commenced, DHDA engaged a probity advisor to develop a probity plan and put in place probity arrangements, including a process for declaring potential conflicts of interest. Declaration of interests for the Seqirus procurement was incomplete. A decision against ‘de-bundling’ the different products favoured Seqirus compared to other potential providers.
Probity planning and documentation
2.51 The Department of Finance advises that an external probity specialist may need to be appointed where: the procurement is high value, complex or unusual; the integrity of the procurement may be questioned; or a prequalified or limited tender process is proposed.78 DHDA engaged AGS as a probity advisor for the contract negotiations from July 2020 to August 2020. In August 2020 AGS finalised a probity plan, protocols for engaging with Seqirus, and collated conflict of interest declarations. AGS attended steering committee and working group meetings (see paragraphs 2.42 to 2.43). As of July 2025 DHDA had not completed any risk assessments that considered probity risks of the procurement.
2.52 In addition to record keeping requirements specified in the CPRs (see paragraph 2.1), DHDA’s records management policy requires staff to create and maintain complete and accurate records of all business activities. The August 2020 probity plan emphasised the importance of making and retaining records to provide evidence that the procurement was properly conducted. DHDA did not retain all probity documents provided by AGS. For example, the declarations of interests report was not retained and in February 2025 DHDA advised the ANAO that it was unable to find copies of the governance and process plan (see paragraph 2.42) and declaration of interests forms that had been provided to it by AGS. In April 2025 AGS re-provided the probity documentation to DHDA to assist with the ANAO audit. Records were not maintained for informal meetings between DHDA and Seqirus on at least 15 occasions between May 2016 and May 2024.
2.53 In November 2020, AGS provided DHDA a probity sign-off letter and declarations of interests report, which stated that there were no unconsidered probity issues and that appropriate steps had been taken for considered issues.
Conflicts of interest management
2.54 The CPRs require officials to act ethically throughout a procurement including recognising and dealing with actual, potential or perceived conflicts of interest.79 DHDA’s conflicts of interest policy: requires that staff declare any conflicts of interest upon engagement with the department and when there has been a change in circumstances or work responsibilities; requires that senior executive service (SES) employees complete an (at a minimum) annual declaration of interests; notes that separate conflict of interest declarations may be required by specific business processes, such as procurement, stating that procurement is considered an area of high risk for conflict of interest; and states that additional requirements may be applied for groups of employees undertaking particular higher risk functions.
2.55 The AGS August 2020 Seqirus probity plan outlined the process for identifying and managing actual or perceived conflict of interests: declarations of conflicts of interest should be a standing item at all meetings; all DHDA officials involved in the procurement and external advisors were required to complete a declaration form, including nil declarations; AGS would monitor declarations; and all declared conflicts should have management strategies with the agreement of the chairs of the steering committee.
2.56 Declarations of interests were incomplete for the Seqirus procurement.80 When interests were declared, a management plan was developed.
- DHDA employees did not complete any conflict-of-interest declarations for the procurement prior to the engagement of AGS in July 2020. By this stage in the procurement, decisions had already been made regarding a limited tender of Seqirus, the negotiation budget and the general structure of the agreement.
- In August 2020 AGS collected 52 declarations from individuals in DHDA; the Department of Finance; the Department of Infrastructure, Transport, Regional Development and Communications; AGS; EY; and KWM.81 Five of the 14 SES officials the ANAO identified as involved in the procurement and/or contract management82 and DHDA’s Secretary83 completed declarations specific to the procurement in the August 2020 AGS process.
- DHDA’s Seqirus contract managers competed conflict-of-interest declarations in December 2024 and January 2025 as part of a separate process. No conflicts were declared. No SES official associated with the contract management of Seqirus completed a declaration specific to the contract management.
- Five of the nine contracted organisations involved in scoping work completed conflict of interest declarations as part of their tender submissions and a further two completed conflict of interest declarations as part of the AGS process. DHDA was unable to locate conflict of interest declarations for two of the nine organisations (KPMG and University of Melbourne). Two declared conflicts (including that EY was the auditor for CSL, the parent company of Seqirus) had a management plan.
Management of gifts, benefits and hospitality
2.57 The CPRs require officials to not accept inappropriate gifts or hospitality.84 DHDA’s 2017 gift and benefits policy stated ‘Employees must declare all gifts which are not an “inconsequential gift or benefit” by completing a gifts and benefits declaration form.’ In December 2019 the policy was updated to specify a threshold of $20 or more for recording the acceptance of gifts and benefits. The 2020 Seqirus probity plan stated that gifts and hospitality should be avoided ‘as much as possible’. DHDA’s gifts and benefits register does not have any declarations related to the Seqirus procurement or contract management.85
Recommendation no.4
2.58 The Department of Health, Disability and Ageing review and improve controls to ensure:
- probity risks, including potential conflicts of interest, are identified and managed from the early stages of procurements and prior to major procurement decisions being made; and
- appropriate records are kept of relevant meetings with parties to a procurement process, which includes contract management.
Department of Health, Disability and Ageing response: Agreed.
2.59 The Department of Health, Disability and Ageing will review and update internal procurement guidance and procedural documentation to improve the identification and management of probity risks and record keeping of procurement related records in line with the Commonwealth Procurement Rules.
Equitable treatment of suppliers
2.60 The CPRs state that all potential suppliers must be treated equitably.86 Ethics in procurement includes ensuring that suppliers should not be excluded from consideration for inconsequential reasons.87 As noted in paragraph 2.10, despite 2018 and 2019 scoping and legal advice stating that a competitive process was feasible and potentially beneficial, and options that included market competition, no competitors to Seqirus were considered as part of the 2020 procurement. The EY analysis stated that bundling the three product categories into a single procurement was described by industry participants as an unfair barrier to competition (see Appendix 5).
Are arrangements for contract management fit for purpose?
DHDA developed a contract management plan, which lacked key elements, including consideration of ongoing contract risk management and planning for contract transition. As of July 2025 DHDA had largely implemented relevant aspects of its contract management plan. DHDA monitors contract performance through regular reporting from Seqirus. Contract performance management could be improved through consideration of the establishment of performance measures addressing quality, cost, responsiveness/timeliness and/or customer satisfaction. DHDA’s contract with Seqirus has provisions to prepare for an influenza pandemic.
2.61 The CPRs and Contract Management Guide state that contract management is important in achieving the objectives of a procurement and value for money.88 Contract management includes day-to-day contract administration and performance management.89
Contract management planning
2.62 DHDA’s contract with Seqirus meets the characteristics of a ‘strategic’ contract as defined by the Contract Management Guide.90 The Contract Management Guide lists six ‘required’ or ‘strongly recommended’ activities during the start-up planning phase for strategic contracts. DHDA’s contract management planning partly aligned with these activities (Table 2.2).
Table 2.2: Assessment of DHDA’s Seqirus contract management planning
|
Contract Management Guide |
Alignment of Seqirus contract management planning |
|
0.1 Assess risk |
▲ |
|
0.2 Develop a contract management plan |
◆ |
|
0.3 Develop a risk management plan |
▲ |
|
0.4 Develop other plans if requireda |
■ |
|
0.5 Consider and manage any contract transition issues |
■ |
|
0.6 Skills development |
■ |
Key: ◆ Aligned ▲ Partially aligned ■ Not aligned
Note a: Other plans may include transition, communication, stakeholder engagement, and probity plans.
Source: Department of Finance, Contract Management Guide, Finance, Canberra, 2020, p. 13 and ANAO analysis of DHDA documentation. The ANAO referenced version 1.5 of the Contract Management Guide (December 2020). The current 2023 version is available from https://www.finance.gov.au/government/procurement/contract-management-guide [accessed 1 July 2025].
2.63 KWM assessed the legal risks of the contract in November 2020, including mitigations such as specific contract clauses that provided protection to the Commonwealth. DHDA completed a risk assessment for the contract between September 2020 and November 2020.91 Five low to medium risks were identified, all of which were accepted. Controls for these risks have been implemented. A contract management plan (see paragraph 2.64) did not assess ongoing contract management risk.
2.64 The Contract Management Guide states that a contract management plan should contain information to ensure that value for money is achieved and which reflects the level of complexity and risk associated with the contract.92 DHDA has two contract management plan templates, including one for strategic/complex contracts. DHDA engaged KWM to write a contract management plan, which it finalised in December 2020 and (as of July 2025) was last updated in February 2021.93 The contract management plan largely aligns with the Contract Management Guide and partly aligns to DHDA’s contract management template for strategic/complex contracts. Missing elements include: stakeholders and their roles and responsibilities such as supplier-specified personnel; contract governance such as internal reporting; supplier access and security; and risk assessment and management. The contract management plan includes no requirement for review. The contract management plan is largely aligned to information contained in the contract.
Recommendation no.5
2.65 The Department of Health, Disability and Ageing improve Seqirus contract management planning by: including a fit for purpose risk management plan that is regularly reviewed and incorporating regular review of the contract management plan.
Department of Health, Disability and Ageing response: Agreed.
2.66 The Department of Health, Disability and Ageing is strengthening the Seqirus contract management framework to ensure it remains robust, responsive and fit-for-purpose throughout the life of the agreement. As part of this work, the department is reviewing and updating the Seqirus contract management plan to incorporate:
- a comprehensive and regularly reviewed risk management plan to support proactive identification, assessment and mitigation of contract risks
- clearly defined roles, responsibilities, and accountabilities to ensure effective oversight and performance management
- a formal schedule for annual review of the contract management plan, with flexibility to undertake additional reviews if required to respond to emerging risks or operational changes.
These enhancements are aligned with the Australian Government Contract Management Guide and reflect the department’s commitment to strong governance, transparency and value for money in managing strategic contracts.
2.67 DHDA’s contract management planning did not consider contract transition. As the lengthy process of researching and scoping alternative sustainable solutions to long-term supply of antivenoms, Q fever vaccine and pandemic influenza vaccines between 2016 and 2019 indicates (see paragraphs 2.4 to 2.12), developing and implementing a novel approach requires significant lead time. Potential transition to a new model will also require consideration of how to ensure security of supply during the transition phase.
Recommendation no.6
2.68 The Department for Health, Disability and Ageing commence planning for the conclusion of the Seqirus contract, to ensure sufficient lead time for consideration and (if determined to be value for money) implementation of alternative models for the sustainable long-term supply of antivenoms, Q fever vaccine and pandemic influenza vaccines.
Department of Health, Disability and Ageing response: Agreed.
2.69 The Department of Health, Disability and Ageing recognises the importance of early and deliberate planning to ensure long-term sustainability, cost-effectiveness and value for money in maintaining continuity of antivenom, Q fever vaccine and pandemic influenza vaccine supply. The department will undertake proactive scoping and planning for the conclusion of the Seqirus contract in line with government policies, processes and desired timeframes.
2.70 Skills development involves an entity considering if it has appropriate resources to manage the contract, assessing if the contract management team has the necessary skills, and organising training to address any shortfalls. DHDA’s contract management planning did not consider skills development.94
Contract administration and monitoring of contract performance
2.71 The Contract Management Guide states that effective contract administration includes accurate recording of contract information and progress; scheduling and minuting meetings and performance reviews; and ensuring supplier reporting aligns with contractual obligations.95
- DHDA and Seqirus are required to meet biannually from the effective date until three years following the commencement of pandemic supply. Following this, DHDA and Seqirus are required to meet at least annually until the end of the 10-year agreement. As of May 2025 all except two meetings were held as required between 2022 and 2024.
- The contract management plan outlines contract administration tasks that are to be completed by DHDA and Seqirus within specified timeframes. As of July 2025 administrative activities were largely undertaken in accordance with timeframes. DHDA did not obtain Workplace Gender Equality Act 2012 compliance letters until the ANAO requested them in February 2025.96
2.72 Performance management involves the actions taken to ensure the goods or services are delivered as required under the contract including performance measurement; assessment and adjustment.97 DHDA’s contract with Seqirus and contract management plan set out levers that can be used to manage underperformance comprising: audit; evaluation; dispute resolution; and termination.
2.73 Performance measures could include quality, quantity, cost, responsiveness and customer satisfaction.98 There are no specific and direct performance measures that go to quality, cost, responsiveness/timeliness or customer satisfaction. The contract and contract management plan outline reporting activities that are meant to enable DHDA to monitor contract performance. Seqirus commences reporting activities once the manufacturing facility is built and has regulatory approval and authorisation. Provision of annual plans, bi-annual progress reports and financial viability reports are linked to payments. The reports require Seqirus to attest and provide supporting evidence that it has been manufacturing antivenoms and Q fever vaccine in accordance with the contract, and that it has met all contractual requirements with regard to pandemic influenza vaccine supplies. These reports and bi-annual meetings comprise the contract performance measures. The reports must meet terms of the agreement, as determined by DHDA, prior to payments being made.
2.74 A 2019 Australian Health Management Plan for Pandemic Influenza outlines Australia’s strategy to manage an influenza pandemic and minimise its impact.99 It has not been updated since 2019 to incorporate lessons from the coronavirus disease 2019 (COVID-19) pandemic.100 DHDA’s 2020 contract with Seqirus contains a Pandemic Readiness and Response Plan, which details actions for DHDA and Seqirus if pandemic influenza vaccine supply is required.
2.75 Among other requirements the contract requires Seqirus to:
- operate in a constant state of pandemic readiness with capability to manufacture and supply sufficient doses of pandemic vaccine to vaccinate every member of the Australian population;
- provide information and undertake studies to facilitate the selection of virus strains to be included in the southern hemisphere vaccine;
- maintain stockpiles of critical and raw materials and a national reserve of influenza vaccine seed against multiple influenza virus strains with pandemic potential;
- maintain year-round the requisite scientific and manufacturing expertise, product registrations and other services required for ongoing product development and pandemic readiness;
- submit to DHDA an annual plan and bi-annual progress reports on pandemic supplies and pandemic preparedness and planning activities; and
- attend pandemic preparedness meetings.
2.76 In the event of a pandemic, Seqirus must ensure there is sufficient pandemic vaccines available to distribute to the Australian population within a specified timeframe. Pandemic preparedness discussions occur in biannual meetings and monthly catch ups between DHDA and Seqirus.
Reporting to government
2.77 DHDA updates the Minister on the contract through meeting briefs, reporting on avian influenza preparedness and the regular budget process. DHDA has provided the government a monthly update on the status of the 2020 contract with Seqirus since December 2021.
3. Moderna procurement
Areas examined
This chapter examines whether the 2022 procurement of manufacturing capability for onshore messenger ribonucleic acid (mRNA) vaccines was effective.
Conclusion
The Department of Health, Disability and Ageing’s (DHDA) and the Department of Industry, Science and Resources’ (DISR) 2022 procurement of onshore manufacturing capability for mRNA vaccines was largely effective. Onshore manufacturing capability to supply mRNA vaccines to Australians was established at an approximate cost of $1.5 to $3 billion. Research was effectively undertaken to identify mRNA manufacturing options and suppliers. Procurement planning occurred late after a decision to procure Moderna Australia Pty Ltd (Moderna) had already been made. Tender evaluation and contract negotiations sought to achieve an economical outcome and were effective. Advice to government was detailed, except that it lacked a clear value for money analysis and conclusion. The procurement was conducted in a manner that was largely consistent with ethical principles. Contract management planning and implementation were largely fit-for-purpose as of July 2025. The contract included provisions for pandemic preparedness. Contract management planning would be enhanced by better planning for the conclusion of the Moderna contract in 2032.
Areas for improvement
The ANAO made one recommendation to the Department of Finance regarding protocols for the invocation of paragraph 2.6 of the Commonwealth Procurement Rules. The ANAO made one recommendation to DISR aimed at ensuring procurement planning and probity planning occurs early before procurement decisions are made.
The ANAO also suggested that DHDA consider whether the application of paragraph 2.6 remains relevant to the ongoing management of its contract with Moderna.
3.1 For the 2022 Moderna procurement, which occurred during the COVID-19 pandemic, the accountable authorities of DHDA and DISR set the Commonwealth Procurement Rules (CPRs) aside under paragraph 2.6 of the CPRs.101
- On 21 May 2021 the Secretary for DISR (DISR Secretary) used paragraph 2.6 of the CPRs on the grounds of human health. DISR stated that it would comply with the Public Governance, Performance and Accountability Act 2013 (PGPA Act) and certain sections of the CPRs: paragraphs 4.4 to 4.8, 4.17 and 4.18 (achieving value for money); paragraphs 5.1, 5.2 and 5.5 to 5.8 (encouraging competition); paragraphs 6.2 to 6.8 (efficient, effective, economical and ethical procurement); paragraphs 7.1 to 7.5 and 7.21 to 7.27 (accountability and transparency)102; and part 8 (procurement risk).
- On 7 December 2021 the Secretary for DHDA (DHDA Secretary) used paragraph 2.6 on the grounds of human health. The DHDA Secretary did not set out alternative requirements for how the procurements were to be conducted. The use was to remain in force until ‘formally revoked’.
- As of July 2025 neither DISR nor DHDA had ‘formally’ revoked the use of paragraph 2.6 for the 2022 Moderna procurement. DISR advised the ANAO in June 2025 that the use of paragraph 2.6 was limited to the specific activity, was not enduring and its use in this instance had ceased. DHDA’s use of paragraph 2.6 continues to apply.
Opportunity for improvement
3.2 The use of paragraph 2.6 during a health emergency such as the COVID-19 pandemic may no longer be appropriate once the health emergency has ended. This may be especially relevant for long-term capability procurements where market dynamics change over time. For example, not revoking the use of paragraph 2.6 means that Moderna contract variations do not need to be (and are not) reported on AusTender (see paragraphs 3.49 and 3.51), which reduces transparency over the arrangements. DHDA could consider whether the application of paragraph 2.6 to the ongoing management of its contract with Moderna remains relevant.
3.3 Regardless of the applicability of the CPRs, DHDA and DISR were obliged to conduct the procurement in a manner that is consistent with section 15 of the PGPA Act, which states:
The accountable authority of a Commonwealth entity must govern the entity in a way that … promotes the proper use and management of public resources for which the authority is responsible.103
The PGPA Act defines ‘proper’ to mean effective, ethical, efficient and economical.
3.4 The Australian Government Contract Management Guide (Contract Management Guide) provides the best practice framework for the establishment and management of contracts (see paragraph 2.2).
Were planning and approach to market effective?
In 2020–21, during the COVID-19 pandemic, DISR and DHDA planned for the ongoing supply of COVID-19 mRNA vaccines. Research included consideration of options, pre-existing arrangements, costs and benefits. In August 2020, DHDA used an open request for information process to obtain market intelligence. In March 2021, DISR and DHDA began negotiating with Moderna to establish an onshore mRNA manufacturing facility for COVID-19 and other vaccines. In May 2021, the government approved finalising negotiations with Moderna and, taking into account the outcomes of negotiations with Moderna, an open approach to market. There was a business plan and draft procurement plan, which defined the goals of the procurement, included some consideration of procurement risk, and estimated the economic benefits. The draft procurement plan was developed after an approach to market had commenced and did not include consideration of any risks related to the unusual parallel procurement process with Moderna. Paragraph 2.6 of the CPRs was used to set the rules aside for the procurement after negotiations with Moderna had commenced. Prior to this time there was no documented consideration of which CPR condition or exemption would apply to justify this limited tender approach. DISR transparently published evaluation criteria for the open approach to market.
Procurement planning
Consideration of pre-existing arrangements, non-procurement alternatives and the market’s capacity to competitively respond
3.5 Coronavirus disease 2019 (COVID-19) emerged in December 2019 and developed into a global pandemic. The CPRs state that when a business requirement arises, officials should consider whether a procurement will deliver the best value for money, taking into account factors such as stakeholder input, obligations and opportunities under other existing arrangements.104
3.6 DHDA advertised a request for information (RFI) on AusTender on 10 August 2020 through which it sought to identify, in response to the COVID-19 pandemic, onshore capacity and capability to supply COVID-19 vaccines and treatments. The RFI sought to identify opportunities to expand, modify or repurpose manufacturing and sought information from organisations with, or likely to seek, Therapeutic Goods Administration (TGA) regulatory approval for a COVID-19 vaccine.
3.7 Following the RFI, a September 2020 Update on Capacity and Capability Stocktake report (stocktake report)105 identified the ability to domestically source each step of Australia’s COVID-19 vaccine supply chain. Among its findings and conclusions the stocktake report identified:
- onshore manufacturing requires longer lead times, higher upfront investment costs in facilities, and a partnership/collaboration to establish, but once established has more secure supply and could scale up quickly (depending on the technology);
- four of the seven types of COVID-19 vaccine being developed, including mRNA vaccines (see paragraph 1.14), had insufficient onshore manufacturing capacity, although mRNA vaccines were likely to have sufficient offshore capacity;
- distribution of both onshore and offshore mRNA vaccine supply was challenging due to very cold temperature storage requirements; and
- COVID-19 pandemic vaccines were likely to be obtained through sourcing a range of vaccine types and stockpiling, rather than onshore manufacturing.
3.8 DISR engaged McKinsey & Company (McKinsey) to complete a Business case for onshore manufacturing of mRNA vaccines in Australia (business case)106, which was finalised in March 2021. The business case considered the benefits of mRNA vaccine technology for various uses including a COVID-19 vaccine. Onshore manufacturing of mRNA vaccines was described as having advantages in relation to speed of meeting demand, efficacy in treating seasonal influenza, and security of supply for new vaccines, amongst other benefits. The business case identified that onshore mRNA manufacturing capacity would require Australian governments to ‘play an active role to attract and accelerate onshoring’ including through financial support. The stocktake report and business case did not consider non-procurement options such as public-private partnerships or a new Commonwealth entity for the manufacture of mRNA vaccines.
3.9 The business case presented two options for the onshore manufacturing of mRNA vaccines:
- go now — an onshore brownfield facility and current technology to manufacture COVID-19 vaccines, for supply within 9–12 months; or
- go later — an onshore greenfield facility and ‘home-grown’ technology to manufacture COVID-19 vaccines, for supply in around three years.
3.10 For the go now option, ‘additional direct government support [would be] required to accelerate the critical path to facility establishment.’ Nine potential brownfield sites were identified, with five able to manufacture vaccines in 12 months. The feasibility, benefits, costs and risks for both options were considered (Table 3.1). Both the go now and go later options had benefits of speed, efficacy, and security of supply, and were estimated to generate up to $1.3 billion in annual gross domestic product.
Table 3.1: Key features of go now versus go later mRNA options, March 2021
|
Go now |
Go later |
|
|
Source: ANAO analysis of business case.
3.11 March 2021 advice to the Minister for Industry, Science and Technology and the Minister for Health and Aged Care recommended:
- establishing a brownfield mRNA manufacturing facility within 24 months through a partnership between several existing companies using technology transfer from a COVID-19 vaccine developer (Pfizer, Moderna or CureVac) (preferred option); or
- developing a greenfield mRNA manufacturing facility within 36 months with a leading vaccine manufacturer and an approach to market process to support Commonwealth co-investment in establishing the facility.
3.12 April 2021 advice prepared for government proposed two ‘longer term’ options, noting that Australia’s short-term needs for a COVID-19 vaccine had been met. The draft advice to government did not recommend one option over the other and stated that both options could be pursued simultaneously, although it was unclear if the market could support two onshore manufacturing facilities of this type.107
- Option 1 — an approach to market for a sovereign mRNA manufacturing capability terminating 30 June 2026.
- Option 2 — entering into detailed negotiations for government investment in Moderna to establish an mRNA vaccine and therapeutics manufacturing facility.
3.13 In June 2021 thrombotic side-effects were associated with the AstraZeneca viral vector vaccine and the Australian Technical Advisory Group on Immunisation (ATAGI) recommended the Pfizer mRNA vaccine for people aged 16 to 60 years old (see paragraph 1.16).
3.14 The CPRs state that when a business requirement arises, officials should consider whether a procurement will deliver the best value for money, taking into account factors such as the market’s capacity to competitively respond to a procurement.108 On the basis of the August 2020 RFI and 64 industry submissions, DISR, DHDA and CSIRO prepared the September 2020 stocktake report. The 64 industry submissions included 13 manufacturers, and ‘seven direct proposals from industry and other entities’. The stocktake report stated that the RFI responses did not cover the entire market and did not include some ‘major operators’. The stocktake report stated that 11 of the 13 responding manufacturers were ‘subscale’ (that is, did not manufacture any vaccines in Australia or had very limited scale at the time). The two remaining manufacturers reported commercial scale that was sufficient to fully manufacture vaccines in Australia, and ‘several’ others reported capacity to cover part of the manufacturing process (that is, might require partnership).
3.15 The March 2021 business case estimated mRNA vaccine manufacturing capabilities and the timeline to commence mRNA vaccine production for eight companies, including Moderna. CureVac, Moderna and Pfizer were the only companies assessed to have mRNA vaccine IP. The March 2021 advice to the Minister for Industry, Science and Technology and the Minister for Health and Aged Care stated that three Australian companies had suitable capability to commence commercial scale mRNA vaccine production and the three leading mRNA vaccines (CureVac, Moderna and Pfizer) had demonstrated success in global manufacturing partnerships. DISR held preliminary discussions with CureVac, Moderna and Pfizer in March 2021. Pfizer was not willing to establish a facility or support technology transfer to an Australian manufacturer. In June 2021 CureVac announced that its COVID-19 candidate vaccine did not pass clinical trials. The April 2021 advice prepared for government stated that Moderna had publicly announced its intention to establish a commercial network in Australia in 2021 and was in the process of preparing a proposal for the Australian Government.
3.16 During negotiations in March 2021 for supply of internationally manufactured vaccines to address Australian needs for the pandemic, Moderna proposed a long-term relationship with the Australian Government around pandemic responses. Negotiations with Moderna to establish a facility began in March 2021 (see paragraphs 3.42 to 3.44).
Specifying procurement goals, risk, value and benefits
3.17 On 11 May 2021 the government announced that, as part of the 2021–22 Federal Budget measure ‘COVID-19 vaccine manufacturing capabilities’, it would be seeking to establish the onshore manufacturing of mRNA vaccines.109 On the same day the government approved: finalising negotiations with Moderna to establish an onshore mRNA manufacturing facility for COVID-19 and other vaccines and, taking into account the outcomes of negotiations with Moderna, an approach to market. On 13 May 2021 the Minister for Health and Aged Care stated that the Australian Government remained in discussions with Moderna in relation to establishing onshore mRNA vaccine manufacturing.110
3.18 Resource Management Guide (RMG) 411 Grants, Procurement and other financial arrangements assists non-corporate Commonwealth entities to identify a grant, procurement or other financial arrangement (see paragraph 2.20).111 As the 2022 Moderna procurement involved an annual ‘pandemic preparedness facility fee’ paid to Moderna to maintain a facility to be owned by Moderna (see paragraph 1.17), the financial arrangement had characteristics of a grant. Entities are required to document the reason for using a particular financial arrangement and apply the relevant framework. In May 2021 DISR’s probity advisor for procurement (see paragraph 3.56) stated that the department should determine whether a grant or procurement activity was required and would need to comply with the CPRs or Commonwealth Grant Rules and Guidelines112 whichever process or processes were selected. Neither DISR nor DHDA documented a rationale for treating the financial arrangement as a procurement.
3.19 DHDA requires its officials to develop a procurement plan for procurements estimated at $10,000 or more. DISR’s procurement policy requires employees to develop a business case when planning a procurement. The business case, detailed in paragraphs 3.8 to 3.10, met some of the planning requirements of DISR’s procurement policy. In July 2021 DISR also developed a draft project plan for establishing an onshore mRNA manufacturing capability (mRNA project plan). The mRNA project plan was not finalised and was drafted after the approach to market, which was open from 21 May 2021 to 16 July 2021 (see paragraph 3.26), and after negotiations with Moderna began in March 2021 (see paragraph 3.42). The draft mRNA project plan included the project background, objectives, scope, schedule, governance arrangements, arrangements for monitoring and control, budget, strategies and product description. The objective of the project was described as successfully undertaking an approach to market and progressing negotiations with Moderna.
3.20 The CPRs state that a thorough consideration of value for money begins by officials ‘clearly understanding and expressing the goals and purpose of the procurement.’113 The draft mRNA project plan defined the procurement objectives and identified several expected benefits.
The objectives of this process are for the successful undertaking of the Approach to Market and to progress negotiations with Moderna. Success for the Approach to Market will be the ability to attract a number of proposals that are fully costed for an end-to-end mRNA pharmaceutical manufacturing capability informing advice to the Government. Further, successful negotiations with Moderna will result in the collection of comparable information to the Approach to Market in order for the Government to make an informed decision with respect to the two parallel processes and subsequent support … The Australian economy will benefit from increases to GDP and increased competitiveness relative to other countries. The Australian population will have enhanced and secure access to vaccines and therapeutics including for pandemic response. Our regional neighbours will benefit by having Australia as a leading supplier of health products. The proposal will also benefit the pharmaceutical and biomedical sectors in Australia, and support the Government’s efforts to develop and maintain a highly skilled workforce.
3.21 The May 2021 onshore mRNA approach to market defined the procurement objective as:
- To ensure priority access to, and reliable delivery of, safe and effective prospective mRNA vaccines and any mRNA therapeutics to the Australian population as soon as they are available, on an ongoing basis;
- To provide security of vaccine supply to address pandemics and other health emergencies into the future; and
- To strengthen Australia’s biopharmaceuticals sector, including through enabling potential translation and commercialisation paths for Australian-based research and development.114
The approach to market stated it was ‘complementary to any current discussions … with relevant mRNA vaccine … owners.’ The government had not determined how many organisations would receive funding when the market was approached. When the government announced the procurement on 11 May 2021 (see paragraph 3.17) the stated objective was ‘to support industry and business response to COVID-19 impacts.’
3.22 The Commonwealth Risk Management Policy and CPRs require risk to be considered when making decisions.115 DHDA’s guidance requires staff to assess risk as early as possible when planning a procurement. DISR’s risk management policy requires a risk management plan to be completed for procurements that are medium or high risk. The overall risk of the procurement was not defined in the draft mRNA project plan or elsewhere. The DISR business case outlined risks associated with the go now and go later options. The draft mRNA project plan identified four low-, medium- and high-rated risks and their planned management. Neither of these included any consideration of risks associated with running an open approach to market in tandem with a negotiation with one supplier. In April 2022 DISR completed an ‘assurance advisory report’, which stated that ‘There is no formal reporting of risks across the taskforce or outlined in the project plans, however risks are considered on an ongoing basis in the conduct of the Taskforce activities.’ and ‘discussions around governance, risk and probity have been considered in all forums.’
3.23 The draft mRNA project plan stated that the risk management plan would be regularly updated and shared with all stakeholders. The draft mRNA project plan stated that DHDA shared some of the responsibility for identifying risks from its perspective and developing risk management plans as appropriate. There is no evidence that the risk management plan was updated or shared. DHDA did not identify or assess risks associated with the procurement until June 2023 (see paragraph 3.73), after the contract with Moderna had been finalised.
3.24 The CPRs state that the expected value of a procurement must be estimated before a decision on the procurement method is made (see paragraph 2.24). Neither the September 2020 stocktake report nor the July 2021 draft mRNA project plan estimated the value of the procurement. The March 2021 business case estimated an Australian Government capital investment of $300 to $400 million for a brownfield facility and $500 to $700 million for a greenfield facility.
3.25 The 2020 CPRs required entities to consider economic benefits to the Australian economy for procurements over $4 million (see paragraph 2.25). The March 2021 business case analysed different scenarios and forecast impacts on gross domestic product and employment, along with other criteria, under the different scenarios.
Approach to market
3.26 DISR advised the ANAO in May 2025 that at a 19 May 2021 meeting, the Minister for Industry, Science and Technology verbally instructed DISR on specifications for an open approach to market, including the length of time the approach to market would be open. The approach to market, titled Proposals to establish an onshore mRNA manufacturing capability (onshore mRNA approach to market), was open from 21 May 2021 to 16 July 2021 and was seeking:
information in the form of fully costed proposals to establish an onshore, population-scale mRNA manufacturing capability, to be fully operational with requisite regulatory approvals/licences within a timeframe of between 12 months (or earlier, if possible) and no later than 3 years from finalisation of an agreement with the Australian Government.116
3.27 The onshore mRNA approach to market stated that it was ‘complementary to any current discussions in which the Australian Government is engaging directly with relevant mRNA vaccine IP owners to establish mRNA manufacturing facilities in Australia.’
Justification of limited tender
3.28 The CPRs and DISR procurement guidance state that for procurements at or above the relevant procurement threshold, limited tender can only be conducted in accordance with conditions listed in the CPRs (see paragraph 2.28). As the procurement was above the threshold, a limited tender approach needed to be justified before commencing negotiations with Moderna in March 2021 (see paragraph 3.42). March 2021 and April 2021 advice to government did not provide advice on limited tender conditions or CPR exemptions and there is no other documented consideration of a limited tender condition or exemption. As noted at paragraph 3.1, the DISR accountable authority used paragraph 2.6 of the CPRs on 21 May 2021 to set aside the CPRs.
3.29 DISR stated that paragraph 2.6 would apply ‘in the event that the approach to market results in a procurement activity.’ This is inconsistent with paragraph 2.7 of the CPRs, which states that a procurement ‘begins when a need has been identified and a decision has been made on the procurement requirement.’ An ‘approach to market’ and negotiations with a potential supplier are procurement activities.
Evaluation plan and risk assessment
3.30 The CPRs state that evaluation criteria must be stated in request documentation to enable the fair and transparent identification, assessment and comparison of submissions (see paragraph 2.35). DISR policy requires evaluation processes to be established prior to publishing tender requests. DISR’s general procurement evaluation methodology and evaluation report template list preferred evaluation criteria: capability, capacity, price, risk and social impact. The onshore mRNA approach to market included ‘evaluation criteria’ aligned with DISR internal procurement guidance. The criteria covered: capability (end-to-end onshore, product range, IP rights, product volume and flexibility and regulatory approvals); capacity; cost; risk management; and wider benefits to the Australian economy. In practice, five ‘dimensions’ were used to assess the proposals that were not identical to the published evaluation criteria but which were largely aligned.117
3.31 The Commonwealth Risk Management Policy, CPRs, and DISR internal guidance all emphasise the importance of engaging with risk in a procurement.118 DISR completed a risk assessment and treatment plan for the onshore mRNA approach to market on 12 May 2021, prior to its opening on 21 May 2021. Six risks were identified and assessed, all of which were rated minor or medium after the application of controls, considered to be within tolerance, and accepted. The one medium risk (timeframes for the approach to market impacting the level of interest and quality of proposals) required a treatment plan under DISR policy. The treatment was engagement with organisations and providing advice to tenderers, and weekly monitoring and review of the risk. There is no evidence that the risk was reviewed on a weekly basis. DISR provided tenderers an industry briefing, published questions and answers on their website, and provided tenderers updates on the progress and outcomes from the approach to market (see paragraph 3.67).
3.32 The risk plan for the approach to market broadly referred to the risk of failure to comply with requirements for procurement, including legal and probity obligations (which was rated minor after treatment). There was no explicit consideration of risks associated with running an approach to market and Moderna negotiation in parallel.
Were tender evaluation and contract negotiations effective and economical?
Fourteen tenders provided a response to the approach to market and were assessed in accordance with a plan and criteria. Moderna did not submit a tender and was included in the assessment as a ‘comparator’ using incomplete and different information to the other proposals. Following the assessment process, which recommended several options, including negotiating with both Moderna and another manufacturer, the government decided to finalise negotiations with both companies. DISR developed a plan and risk assessment for the negotiations and engaged experts. Advice to government was detailed, except that it lacked a clear value for money analysis and conclusion. The options had divergent risks and costs, and the final advice to government was to pursue both options. The government decided to establish an arrangement solely with Moderna at an approximate cost of $1.5 to $3 billion. Following this decision, the Moderna facility establishment agreement and expenditure was appropriately developed, authorised and supported by legislation. In the event of a pandemic, DHDA has the option to order any candidate vaccine developed by Moderna for the relevant infection, after which the parties are required to promptly enter into an advance purchase agreement for pandemic supplies, subject to the negotiation of product price and operational terms.
3.33 When setting aside the CPRs on 21 May 2021 DISR stated that it would comply with certain sections of the CPRs (see paragraph 3.1). These included requirements to achieve value for money. The CPRs state that value for money considerations must include financial and non-financial costs and benefits including quality of the goods and services, fitness for purpose of the proposal, experience and performance history, and whole-of-life costs.119
Achievement of value for money through tender evaluation
3.34 The onshore mRNA approach to market received 14 responses, including from state governments, small to medium enterprises and consortiums. Moderna did not submit a response. Timeframes for establishment of a facility ranged from one to three years.
3.35 In May 2021 an Expert Advisory Group (EAG) was established. The EAG comprised seven members who had experience in pharmaceuticals and the manufacturing of medical products, and representatives from CSIRO, DHDA and DISR. The EAG met fortnightly while the approach to market was open and weekly after it closed. McKinsey was engaged to support the EAG during the tender evaluation process.120
3.36 On 19 July 2021 the EAG finalised a methodology to consider the onshore mRNA approach to market responses. The work was conducted in four stages: screening; analytical report; respondent clarification; and assessment.
- Stage 1 Screening — This involved checking responses against the minimum requirements of the approach to market. Three of 14 responses did not pass screening.
- Stage 2 Analytical report — McKinsey prepared an analytical report with an indicative assessment rating against the five dimensions (see paragraph 3.30), and comparing the strengths, limitations and uncertainties of the responses. Responses were sorted into five groups based on whether the proposals had end-to-end manufacturing capability and/or mRNA IP. Two suppliers submitted end-to-end manufacturing proposals with mRNA IP in development. No suppliers had approved mRNA vaccine IP. The approach to market identified no new sources of onshore manufacturing capability.
- Stage 3 Respondent clarification / additional information — The EAG sought clarification from four suppliers on their tenders.
- Stage 4 Qualitative holistic and comparative assessment — The EAG methodology stated that a numerical scoring approach would not be used as it could lead to ‘perverse outcomes’. Responses would be determined by the EAG to be ‘suitable’ or ‘not suitable’ for further consideration by government. Five dimensions, each weighted equally, were used to assess the proposals (see paragraph 3.30). Qualitative considerations comprised pros, cons, trade-offs, risks and opportunities; ability to deliver on the government’s overall objectives; degree of Australian Government control of the facility’s outputs to target priorities for the health and wellbeing of Australians; capacity to contribute to and support global leadership; overall maturity of the organisation and/or consortia; and governance and management arrangements.
3.37 On 6 August 2021 the EAG, assisted by McKinsey, completed a final report assessing the tenders for the onshore mRNA approach to market (assessment report). Moderna was included as a ‘comparator’ in the assessment report and assessed against the five dimensions. Moderna was further compared against shortlisted suppliers with end-to-end manufacturing capability and mRNA IP on the cost of site development, ongoing operations, vaccine purchasing, mRNA IP, and contract structure. To conduct the comparison, the EAG relied on Moderna’s publicly available information and information provided by Moderna to DISR and DHDA during discussions about supply of internationally manufactured vaccines to address Australian needs for the COVID-19 pandemic (see paragraphs 3.42 and 3.44). The assessment report stated that, because of this, the available information on Moderna was less complete (particularly on benefits and costings).
3.38 The assessment report concluded that:
- one tenderer from the approach to market was identified as ‘potentially viable’ for end-to-end onshore manufacturing capability; and
- Moderna’s proposal from its direct negotiations with DISR and DHDA had advantages related to having a commercialised COVID-19 vaccine, mRNA IP and proven experience.
3.39 On 25 August 2021 the government approved entering into negotiations with the ‘potentially viable’ tenderer, alongside ongoing negotiations with Moderna. Advice to the Minister for Industry, Energy and Emission Reductions on 15 October 2021 summarised and appended the EAG final report and listed five options for an onshore mRNA facility, which included procuring either supplier or both. On 25 October 2021 the Minister for Industry, Energy and Emissions Reduction agreed for DISR to notify the remaining 13 respondents to the approach to market of the outcome.
Achievement of value for money through contract negotiations
3.40 DISR drafted a project plan and a governance and process plan (negotiation plan) for the negotiation phase in September 2021.The negotiation plan outlined the objective, scope, schedule, governance arrangements, monitoring and control arrangements; budget, quality strategy, stakeholder engagement strategy and risk management strategy; and assessed risks. Experts were engaged to inform the contract negotiations.121
- Commercial negotiator — From September 2021 to March 2022, DISR engaged Mr Robin Bishop, who undertook the work on a voluntary basis, as a commercial negotiator.
- Commercial advisor and due diligence — In late August 2021 DISR engaged McKinsey as a commercial advisor. In September 2021 Lazard Australia Pty Ltd (Lazard) was engaged as the commercial advisor at the request of the commercial negotiator.122 Lazard analysed the ‘potentially viable’ tenderer’s and Moderna’s financial proposals. In March 2022 Lazard concluded that the Commonwealth was ‘provided with all material commercial and financial advice required to properly consider and fully analyse its transaction risks and purchase options.’
- Strategic advisor — McKinsey assisted in drafting the negotiation mandate and co-ordinating the various advisors’ activities.123
- Legal advisor — King & Wood Mallesons (KWM) was engaged as a legal advisor during the contract negotiation process.124
3.41 A deputy secretary group (DSG), comprised of deputy secretaries from DISR, DHDA, and the Department of Finance, was formed to oversee the negotiation process and engage with state governments. The DSG was responsible for approving negotiation strategies, reviewing options to maximise value for money and providing advice to government. The commercial negotiator, commercial advisor, legal advisor and probity advisor (see paragraph 3.56) attended DSG meetings as required. The DSG met twice weekly125 from August 2021 to April 2022. Meeting minutes were not prepared, however key actions were recorded. The DSG provided the Minister for Health and Aged Care and the Minister for Industry, Science and Resources with updates between October 2021 and March 2022.
Negotiations with Moderna
3.42 Negotiations between DISR, DHDA and Moderna occurred from March 2021 to March 2022. Moderna wished to establish its own facility in Australia, rather than license its IP to a third-party manufacturer. It was estimated that it would take three years to establish a facility. Goals of the negotiation strategy included to establish security of supply for mRNA vaccines in Australia and establish an mRNA ‘ecosystem’ in Australia (including Australian clinical trials, Australian supply chains, a majority Australian workforce, and a research fellowship).
3.43 The negotiations involved considerable back and forth between the two parties on pricing and the health technology assessment process. Moderna did not want vaccine prices to be determined by the Pharmaceutical Benefits Advisory Committee (PBAC). On 9 March 2022 the Minister for Health and Aged Care and Minister for Industry, Energy and Emissions Reduction sought approval from the Prime Minister for a ‘bespoke’ health technology assessment process for Moderna. On 17 March 2022 the Prime Minister approved the bespoke process, noting concern with Moderna’s refusal to participate in the National Immunisation Program126 process. Moderna advised the ANAO in September 2025 that it was concerned about the typical length of the standard pricing process for vaccines in Australia and that its health technology assessment process follows PBAC guidelines.
3.44 The expenditure was appropriately authorised and supported by legislation; legal risks were assessed; and contract indemnity aligned with Australian Government guidance.
Negotiations with ’potentially viable’ tenderer
3.45 Negotiations with the ‘potentially viable’ tenderer occurred from late September 2021 to November 2021. The negotiations considered construction of a facility, time required to achieve end-to-end manufacturing, pricing of the proposal, payment schedules, technology transfer, and the local mRNA ‘ecosystem’.
Procurement outcomes
3.46 In November 2021 DHDA and DISR advised the government on the advantages and disadvantages of the Moderna and ‘potentially viable’ tenderer options, noting that the proposals were divergent in cost and risk. DHDA and DISR advised government to pursue both options, noting that the ‘potentially viable’ tenderer option brought ‘unique’ benefits at a low marginal cost. DHDA’s and DISR’s advice did not include further consideration of value for money, stating that this would be considered after additional analysis. A clear value for money conclusion was not expressed in any later documentation relating to the procurement.
3.47 In December 2021 the government approved the establishment of a Moderna mRNA manufacturing facility and did not approve progressing with the ‘potentially viable’ tenderer. On 14 December 2021 the Prime Minister, Minister for Finance, Minister for Health and Aged Care, and Minister for Industry, Energy and Emissions Reduction announced that a new sovereign vaccine manufacturing facility would be built in Australia by Moderna.127 On 24 March 2022 the Prime Minister, Minister for Finance, and Minister for Health and Aged Care announced that the agreement with Moderna had been finalised.
3.48 The Minister for Health and Aged Care signed the facility establishment agreement with Moderna on 24 March 2022128, valued at an approximate cost of $1.5 billion to $3 billion. In December 2024 a DHDA deputy secretary signed the non-pandemic supply agreement with Moderna, a sub-agreement to the facility establishment agreement (see paragraph 1.19). The facility establishment agreement involved an annual pandemic preparedness facility fee and an annual minimum purchase commitment of COVID-19 vaccines and other respiratory mRNA vaccines. The agreement ends on 24 August 2032.
3.49 As outlined in paragraph 3.21, the objectives of the procurement were to: ensure ongoing priority access to and reliable delivery of mRNA vaccines to the Australian population as soon as they become available; provide security of vaccine supply in future pandemics and health emergencies; and strengthen Australia’s biopharmaceuticals sector including through commercialisation paths for Australian-based research and development. The facility establishment agreement requires Moderna to: establish onshore mRNA manufacturing capability in Australia to assist with pandemic readiness and for non-pandemic respiratory viral infections; and support a ribonucleic acid ‘ecosystem’ in Australia through a minimum spend towards initiatives supporting research and development. Moderna must keep the government apprised of its global pipeline of mRNA respiratory infection vaccines, which the government can choose to purchase.
3.50 As summarised at Table S.2, to exercise its option to purchase pandemic vaccines, the government must provide written notice to Moderna within a designated timeframe. Upon exercising its option, the parties must promptly enter into an advance purchase agreement, subject to the negotiation of product price and operational terms, which is to be conducted outside the standard National Immunisation Program process (see paragraph 3.42).
AusTender reporting
3.51 Because paragraph 2.6 was invoked for the Moderna procurement, DHDA was not required to and did not report the contract on AusTender (see paragraph 2.50). While the use of paragraph 2.6 is in effect, contract variations also do not require being reported on AusTender, and no variations have been reported on AusTender.
3.52 The Department of Finance’s RMG 423 Procurement Publishing and Reporting Obligations states that ‘when a relevant entity undertakes a limited tender (that is not an open tender) valued at or above the relevant threshold, the justification for the limited tender must also be reported on [AusTender]’. The justifications available include that paragraph 2.6 was applied in some part.129 As DHDA did not report the contract on AusTender, it also did not publish the use of paragraph 2.6.
3.53 Senate Order 13 requires Australian Government entities to publish information twice annually about contracts with a total value of $100,000 or more. Senate Order 13 states that this may include a published link to a complying report on AusTender. RMG 403 Meeting the Senate Order for Entity Contracts130 directs entities to refer to AusTender for contract information. RMG 403 allows for certain contractual arrangements to be excluded from publication, for example if disclosure is contrary to public interest. Neither Senate Order 13 nor RMG 403 explain reporting requirements for contracts exempt from AusTender reporting. DHDA’s contract with Moderna is a ten-year contract (see paragraph 3.48). DHDA advised the ANAO in April 2025 that, as the Moderna contract is exempt from reporting on AusTender under paragraph 2.6 of the CPRs, it is not published on the departmental website under Senate Order 13.
Recommendation no.7
3.54 The Department of Finance improve guidance to Australian Government entities on:
- the appropriate use of paragraph 2.6 of the Commonwealth Procurement Rules, including how it should be used and revoked for short- and long-term procurements; appropriate timing for its use; and requirements for how its use should be reported on AusTender; and
- how contracts exempt from AusTender reporting should be dealt with under Senate Order 13.
Department of Finance response: Agreed.
3.55 The Department of Finance (Finance) will update its procurement guidance regarding the appropriate use of paragraph 2.6 of the Commonwealth Procurement Rules. Finance will also update its guidance on how entities should report procurement contracts in their Senate Order 13 submissions, where those contracts have been exempted from reporting on AusTender.
Were procurement activities conducted ethically?
Probity advisers developed probity plans and managed risk. Probity advisors oversaw the management of conflicts of interest. The declaration of interests for the Moderna procurement was incomplete in both DHDA and DISR. DHDA employees declared gifts and benefits appropriately; DISR employees did not. Ethics in procurement includes the equitable treatment of tenderers. There was transparency over the parallel process of conducting an open approach to market while finalising negotiations with one supplier. No tenders were removed from consideration for inconsequential reasons and all tenderers were appropriately informed throughout the process.
Probity planning
3.56 The Department of Finance advises that an external probity specialist may need to be appointed where the procurement is high value, complex or unusual; the integrity of the procurement may be questioned; or a prequalified or limited tender process is proposed (see paragraph 2.51). DISR engaged Maddocks from May 2021 to December 2021 as probity advisor for the approach to market processes.131 DHDA engaged Sententia Consulting (Sententia) from August 2021 to April 2022 as probity advisor during contract negotiations.132 Although DISR and DHDA engaged probity advisors for the approach to market and negotiation phases of the 2022 Moderna procurement, there was no probity advisor in place when decisions were made about simultaneously approaching the market and negotiating with Moderna. Maddocks’ May 2022 final probity report stated that the role of Maddocks did not include advising on the proposed methodology for approaching the market to establish mRNA capability.
3.57 Maddocks and Sententia developed probity plans.133 The July 2021 Maddocks probity plan outlined the management of confidentiality, record keeping, complaints, privacy, fraud, conflicts of interest, and gifts and benefits. Maddocks suggested the development of a probity plan after McKinsey sought approval to assist state governments interested in submitting a proposal in response to the approach to market (Table 3.2). The September 2021 Sententia probity plan covered PGPA Act requirements, conflict of interest declarations, confidentiality, interactions with tenderers, and records management obligations.
3.58 DISR considered general probity risk and controls in risk assessments. DHDA did not complete any risk assessments, including of probity risks, during the procurement.
3.59 Maddocks did not identify any unresolved probity issues in its May 2022 probity report. Sententia’s November 2022 probity report concluded that ‘the negotiation process for the agreements was undertaken in a manner that was broadly consistent with the approved Probity Plan’ and ‘that the negotiation process undertaken by the Departments provided a defensible basis for the entering of the Moderna … [agreement] by Government.’
Recommendation no.8
3.60 The Department of Industry, Science and Resources implement procurement planning, including probity plans, at the early stages of procurements prior to decisions being made that will determine the direction and influence the outcome of the procurement.
Department of Industry, Science and Resources response: Agreed.
3.61 The Department acknowledges the importance of early planning, including probity considerations and has implemented several improvements over the past few years. Since 2022, the Department has invested significant time and effort to improve the skills and capabilities of our staff. This includes the establishment of an Integrity Branch, improved guidance and increased support for procurement projects, and improved systems and practices.
Conflicts of interest management
3.62 The CPRs require officials to act ethically throughout a procurement including recognising and dealing with actual, potential or perceived conflicts of interest. DHDA’s conflicts of interest policy also establishes requirements for specific business processes such as procurements and for senior executive service (SES) employees (see paragraph 2.54). DISR’s 2020 conflict of interest policy required staff to undertake awareness training on conflicts of interest upon engagement and annually. In October 2022 the DISR policy was updated to include that staff may be required to complete a conflict-of-interest declaration for specific activities. It gives as an example that staff involved in procurement activities should complete activity-based conflict of interest declarations (regardless of whether there is a conflict to disclose) commensurate with risk.134 DISR SES officers are required to complete a declaration of personal interests form annually or if there is a change of work responsibilities, regardless of whether they have a conflict to disclose.
3.63 Maddocks’ probity plan outlined the requirement to disclose conflicts of interest (including nil declarations) to an executive prior to accessing any information about the procurement, if an equivalent declaration had not already been made, and regularly updating declarations. Conflicts were to be declared at the commencement of each meeting of the EAG and prior to considering any proposals. DISR maintained a conflict of interest register and the signed declarations. Sententia’s probity plan advised that all project participants and contracted organisations complete conflict-of-interest declaration forms (including nil declarations).135 Sententia indicated that it would maintain a register and related treatment plans for declared conflicts.
3.64 The management of conflicts of interest is described in Table 3.2. Procurement-specific declarations of interest (including nil declarations) were not consistently completed by DISR and DHDA non-SES and SES employees involved in the procurement. One EAG member declared a conflict relating to 17 years employment with the ‘potentially viable’ tenderer, which was considered insignificant resulting in no management plan being developed.
Table 3.2: Management of conflicts of interest for 2022 Moderna procurement
|
Relevant party |
Declaration and management of interests |
|
Expert Advisory Group members |
All EAG members made declarations regarding potential conflicts during the procurement process, as required. An EAG member worked as an executive for the ‘potentially viable’ tenderer from 2002 to 2019, including working at one of their manufacturing facilities. The conflict was declared, however there was no discussion of the conflict at EAG meetings or a management plan. DISR’s advice to the Minister for Industry, Science and Technology in May 2021 recommending this person for the EAG noted that the person’s ‘previous employment was noted but not considered significant to exclude [them] from … discussions. DHDA’s experience is that [they are] extremely balanced when dealing with [the ‘potentially viable’ tenderer].’ DISR advised the ANAO in June 2025 that it considered the conflict to be ‘low risk’ and appropriately treated. No treatment plan was developed. Other conflicts were discussed at EAG meetings and recorded on DISR’s conflict of interest register, and noted with no further action to be taken. |
|
DISR employees |
Eight of the 15 non-SES DISR staff that the ANAO identified to be involved in the Moderna procurement submitted declarations in 2021. No conflicts were declared. No non-SES staff have completed a declaration since 2021. One potential conflict was recorded on DISR’s conflict of interest register. It was noted with no further action to be taken. All four DISR SES employees that the ANAO identified as relevant to the procurement submitted personal interest declaration forms in line with departmental policies.a One SES declared shares in the ‘potentially viable’ tenderer in 2020; no management plan was developed. One of the four submitted a declaration specific to the procurement. DISR’s Secretary provided an annual declaration to the Minister for Industry, Science and Technology from 2020 to 2025. |
|
DHDA employees |
Seven of 20 DHDA staff that the ANAO identified as relevant to the procurement, contract negotiations and/or contract management submitted conflict of interest declarations in 2021 or later. DHDA’s Moderna contract managers have not made a declaration. DHDA put in place a management plan for a declared conflict. One potential conflict from a DHDA employee was recorded on DISR’s conflict of interest register. It was noted with no further action to be taken. All three DHDA SES staff the ANAO identified as relevant to the procurement, contract negotiations and/or contract management submitted personal interest declaration forms in line with departmental policies. None of the three submitted a declaration specific to the procurement, contract negotiations and/or contract management. The DHDA Secretary provided a declaration to the Minister for Health and Ageing on three occasions since the contract was signed in 2022: in August 2023, July 2024 and February 2025. |
|
Contractors |
In September 2021 all organisations contracted during procurement planning, the approach to market and/or contract negotiations, and Mr Robin Bishop, completed conflict of interest declarations, except for McKinsey. Declared conflicts were not considered to be material or were assessed as low risk. McKinsey declared that it was not aware of any known, actual or potential conflicts of interest but declined to disclose other clients that may cause actual, perceived or potential conflicts. In June 2021 DISR’s conflict of interest register listed one conflict relating to McKinsey, involving a separate team at McKinsey wanting to assist state governments with a response for the mRNA approach to market. Maddocks advised DISR that the most effective way to avoid probity risk was for McKinsey not to be involved in related activities but noted that ‘the department is seeking other options for how these risks can be managed.’ Maddocks and Sententia proposed controls to mitigate risks, which they noted were accepted and implemented by DISR. |
Note a: One DISR SES official was also a member of the EAG. In the table, this official’s management of conflict of interest is considered once in relation to the EAG.
Source: ANAO analysis.
Gifts, benefits and hospitality
3.65 The CPRs require officials to act ethically throughout a procurement including not accepting inappropriate gifts or hospitality. DHDA’s gifts and benefits policy requires employees to record any gift or benefits valued over $20 or more which they have accepted (see paragraph 2.57). DISR’s gifts and benefits policy requires employees to record gifts or benefits of over $100 that they have accepted. Maddock’s probity plan outlined that personnel must not seek or receive any gifts or benefits from tenderers and must notify the appropriate delegate if offered a gift or benefit. Sententia’s probity plan advised that all project participants avoid gifts and benefits as much as possible.
3.66 DHDA and DISR maintain a gifts and benefits register. In March 2022 a DISR senior executive suggested they could ask Lazard to organise drinks after the contract was signed. A dinner, valued at over $100, was offered to five DHDA and 10 DISR employees. The dinner invitation was declared by DHDA officials (including one who accepted the offer) and not by the four DISR officials who accepted the offer, despite being directed to do so by a DISR deputy secretary.136
Equitable treatment of suppliers
3.67 The CPRs state that all potential suppliers must be treated equitably (see paragraph 2.60). Suppliers who submitted tenders to the approach to market were given sufficient opportunities for feedback/debriefing. DISR held an online industry briefing on 27 May 2021, which DISR reported over 100 people registering to attend. DISR published the industry briefing on its website.137 In response to enquiries during the approach to market, DISR published questions and answers on its website. DISR maintained a communications register during the approach to market from May to December 2021, including meetings and emails with potential tenderers. DISR declined the ‘potentially viable’ tenderer’s requests to enter into direct discussions outside the approach to market. Unsuccessful suppliers were informed of the approach to market outcomes in December 2021. All eight suppliers that requested feedback were provided feedback in December 2021 and January 2022. One supplier commented during its feedback session that eight weeks to prepare an approach to market submission was short, however there were no formal complaints that this created a barrier to competition. There was no evidence that the screening and assessment processes removed tenders from consideration for inconsequential reasons.
3.68 The process of an open approach to market while simultaneously undertaking negotiations with one supplier (Moderna) carried risk of perceived or actual inequitable treatment of suppliers. Sententia noted in its November 2022 probity report that:
[Maddocks] raised with the [deputy secretary group] the risks related to the transparency and integrity of the parallel processes and consider that these risks were understood by [deputy secretary group] members. While the parallel negotiations added to the complexity of the negotiations, the [deputy secretary group] maintained awareness of the status of each respective process and were sensitive to stakeholder risks in the interaction of the overlapping processes. This was particularly evident in the management of any public announcements regarding the progress of the negotiations or the [approach to market] process … there was adequate separation between the matters during the negotiation process, and we did not observe any instances of discussions with Moderna or [the ‘potentially viable’ tenderer] regarding the supply contracts for COVID-19 vaccines inappropriately influencing the negotiation process.
3.69 There was transparency over the parallel process. As set out at paragraph 3.17, on 11 May 2021 the Minister for Health and Aged Care publicly announced that the Australian Government was in active negotiations with Moderna to establish onshore mRNA vaccine manufacturing. The approach to market stated that it was ‘complementary to any current discussions in which the Australian Government is engaging directly with relevant mRNA vaccine IP owners to establish mRNA manufacturing facilities in Australia’ (see paragraphs 3.26 and 3.27). The EAG assessed Moderna’s proposal against the proposals submitted in response to the approach to market (see paragraphs 3.37 and 3.38). DISR engaged a commercial negotiator (see paragraph 3.40).
3.70 Sententia noted that there were instances of bilateral discussions during the contract negotiations which were not documented. This included phone calls between the commercial negotiator and representatives of tenderers. Sententia stated that these meetings raised the risk that ‘individuals may have acted in a manner that was inconsistent to the interests of the Commonwealth in negotiating certain positions taken in agreements.’ Sententia’s report stated that it was aware of communication between Ministers, Ministers’ offices and tenderers. Sententia noted that the probity plan was provided to Ministers’ offices and that ‘the Departments actively sought to coordinate and advise on communication between ministers and proponents to manage consistency with the negotiation strategy.’
Are arrangements for contract management fit for purpose?
DHDA has completed contract risk assessments and updates the government on risks and issues. There is a contract management plan, relevant aspects of which DHDA has largely implemented as of July 2025. Contract performance management could have been enhanced through the establishment of specific and direct performance measures that go to quality, cost, responsiveness and/or customer satisfaction. DHDA’s contract with Moderna has provisions to require Moderna to prepare for a pandemic.
3.71 The CPRs and Contract Management Guide state that contract management is important in achieving the objectives of a procurement and value for money (see paragraph 2.61). DHDA is responsible for the ongoing management of the contract with Moderna.
Contract management planning
3.72 DHDA’s contract with Moderna meets the characteristics of a ‘strategic’ contract as defined by the Contract Management Guide.138 The Contract Management Guide lists six ‘required’ or ‘strongly recommended’ activities during the start-up planning phase for strategic contracts. DHDA’s contract management planning aligned with some of these activities (Table 3.3).
Table 3.3: Assessment of DHDA’s Moderna contract management planning
|
Contract Management Guide |
Alignment of Moderna contract management planning |
|
0.1 Assess risk |
◆ |
|
0.2 Develop a contract management plan |
◆ |
|
0.3 Develop a risk management plan |
◆ |
|
0.4 Develop other plans if requireda |
▲ |
|
0.5 Consider and manage any contract transition issues |
▲ |
|
0.6 Skills development |
■ |
Key: ◆ Aligned ▲ Partially aligned ■ Not aligned
Note a: Other plans may include transition, communication, stakeholder engagement, and probity plans.
Source: Department of Finance, Contract Management Guide, Finance, Canberra, 2020, p. 13 and ANAO analysis of DHDA documentation. The ANAO referenced version 1.5 of the Contract Management Guide (December 2020). The current 2023 version is available from https://www.finance.gov.au/government/procurement/contract-management-guide [accessed 1 July 2025].
3.73 There were risk assessments and management in relation to DHDA’s contract with Moderna, including KWM assessment of the legal risks of the facility establishment agreement (March 2022); a DISR risk assessment for implementation of the Moderna agreement including facility construction (September 2022); and a DHDA risk register for the contract (June 2023). The risk register identified eight risks, including one high-rated risk (‘Moderna unable to obtain approvals for the facility or vaccines’), which was accepted by the risk owner. The draft contract management plan contains a risk management strategy with references to the KWM legal risk assessment and the risk register. The government has been updated on project risks monthly since June 2024 (see paragraph 3.83).
3.74 The Contract Management Guide states that a contract management plan should contain information to ensure that value for money is achieved and which reflects the level of complexity and risk associated with the contract. DHDA has two contract management plan templates, including one for strategic/complex contracts (see paragraph 2.64). DHDA finalised the first version of a contract management plan for the Moderna agreements on 10 July 2023, which was updated in June 2025. The contract management plan is largely aligned with the Contract Management Guide and DHDA’s contract management template for strategic/complex contracts. Missing elements include: contract governance such as internal reporting and contract review; risk reporting and escalation processes; and details of the performance guarantee (see paragraph 3.79).
3.75 DHDA also has an assurance plan and a draft stakeholder engagement plan. DHDA advised the ANAO in April 2025 that it follows enterprise-wide fraud control and security plans for the contract with Moderna. As of April 2025 DHDA had not finalised a supply chain plan as vaccine supply has not yet commenced.
3.76 DHDA has not developed a transition plan for the end of the contract. The contract management plan provides a list of four ‘good practice’ transition activities to be implemented at the end of the agreement.
3.77 Skills development involves an entity considering if it has appropriate resources to manage the contract, assessing if the contract management team has the necessary skills, and organising training to address any shortfalls. The contract management planning did not consider skills development.
Contract administration and monitoring of contract performance
3.78 The Contract Management Guide states that effective contract administration includes accurate recording of contract information and progress; scheduling and minuting meetings and performance reviews; and ensuring supplier reporting aligns with contractual obligations.139
- DHDA, Moderna and other stakeholders are required to hold progress meetings to discuss progress of the facility build amongst other matters, which have been held as required.
- An Intergovernmental Steering Committee (IDC) was formed to consider facility build progress, regulatory approvals and delivery of Moderna reporting, including for the mRNA ‘ecosystem’ (see paragraph 3.42). The IDC includes members from DISR, DHDA, and mRNA Victoria. Occasionally the TGA attended meetings. The IDC has met three or four times a year between September 2022 to May 2025.
- The contract management plan outlines contract administration tasks that are to be completed by DHDA and Moderna within specified timeframes. As of July 2025, administrative activities were largely undertaken in accordance with timeframes.
3.79 Performance management involves the actions taken to ensure the goods or services are delivered as required under the contract including performance measurement; assessment and adjustment (see paragraph 2.72). DHDA’s contract with Moderna and contract management plan have performance incentives and levers that can be used to manage underperformance comprising: audit, evaluation, dispute resolution and termination. DHDA and Moderna Inc. signed a performance guarantee in March 2022.
3.80 Performance measures could include quality, quantity, cost, responsiveness and customer satisfaction (see paragraph 2.73). There are no specific and direct performance measures that go to quality, cost, responsiveness/timeliness or customer satisfaction. The contract and contract management plan outline reporting activities to enable DHDA to monitor contract performance. The reports require Moderna to attest to compliance with contractual obligations including the products supplied, and identify any issues that may prevent Moderna from meeting its obligations.
3.81 The facility establishment agreement requires Moderna to draft, maintain and comply with a pandemic risk management plan that outlines the operational requirements for Moderna in the event of a pandemic. Moderna finalised the first version of the plan in November 2022 and updated it in response to comments from the government in February 2023. The pandemic risk management plan outlines the operational processes in the event of a pandemic. Although it is not a requirement under the contract, DHDA advised the ANAO in April 2025 that a half day pandemic preparedness workshop was held with Moderna in April 2025. As of April 2025 DHDA had not planned further meetings with Moderna to discuss pandemic preparedness.
3.82 If a pandemic is declared and the Australian Government enters into the pandemic advance purchasing agreement with Moderna (see paragraph 3.50), the agreement contains a vaccine dose delivery schedule that will apply subject to the successful development and approval of a pandemic vaccine.
Reporting to government
3.83 DHDA provided reporting to government on the Moderna facility contract quarterly from March 2023 to March 2024 and monthly since June 2024. Reporting from March 2023 to June 2024 commented on the progress of the facility build and other deliverables under the agreements. Reporting since June 2024 provided an overall status and an update on project risks and issues.
Appendices
Appendix 1 Entity responses
Department of Health, Disability and Ageing


Department of Industry, Science and Resources

Department of Finance

Moderna Australia Pty Ltd




Appendix 2 Improvements observed by the ANAO
1. The existence of independent external audit, and the accompanying potential for scrutiny improves performance. Improvements in administrative and management practices usually occur: in anticipation of ANAO audit activity; during an audit engagement; as interim findings are made; and/or after the audit has been completed and formal findings are communicated.
2. The Joint Committee of Public Accounts and Audit (JCPAA) has encouraged the ANAO to consider ways in which the ANAO could capture and describe some of these impacts. The ANAO’s corporate plan states that the ANAO’s annual performance statements will provide a narrative that will consider, amongst other matters, analysis of key improvements made by entities during a performance audit process based on information included in tabled performance audit reports.
3. Performance audits involve close engagement between the ANAO and the audited entity as well as other stakeholders involved in the program or activity being audited. Throughout the audit engagement, the ANAO outlines to the entity the preliminary audit findings, conclusions and potential audit recommendations. This ensures that final recommendations are appropriately targeted and encourages entities to take early remedial action on any identified matters during the course of an audit. Remedial actions entities may take during the audit include:
- strengthening governance arrangements;
- introducing or revising policies, strategies, guidelines or administrative processes; and
- initiating reviews or investigations.
4. In this context, the below actions were observed by the ANAO during the course of the audit. It is not clear whether these actions and/or the timing of these actions were planned in response to proposed or actual audit activity. The ANAO has not sought to obtain assurance over the source of these actions or whether they have been appropriately implemented.
- In December 2024 and January 2025, the Department of Health, Disability and Ageing’s (DHDA) Seqirus contract managers completed conflict of interest declarations (see paragraph 2.56).
- In February 2025 DHDA obtained Workplace Gender Equality Act 2012 letters from Seqirus (see paragraph 2.71), a requirement under the 2020 contract.
- In February 2025 DHDA advised the ANAO that DHDA employees now complete a conflict of interest declaration for every new or varied procurement (see paragraph 2.54).
- In May 2025 the Department of Industry, Science and Resources (DISR) updated its gifts and benefits register (see paragraph 3.66).
- In June 2025 DHDA advised the ANAO that it was updating the Seqirus contract management plan (see paragraph 2.64).
- In June 2025 DHDA drafted version 1.3 of the Moderna contract management plan (see paragraph 3.74).
- In June 2025 DISR advised the ANAO that it had referred the matter of a staff member requesting a benefit from Lazard to its Integrity Branch, that it was changing its gifts and benefits declaration process, and that it was reviewing its conflict of interest policy (see paragraph 3.62).
Appendix 3 Timeline of Seqirus procurement
Appendix 3 Timeline of Seqirus procurement
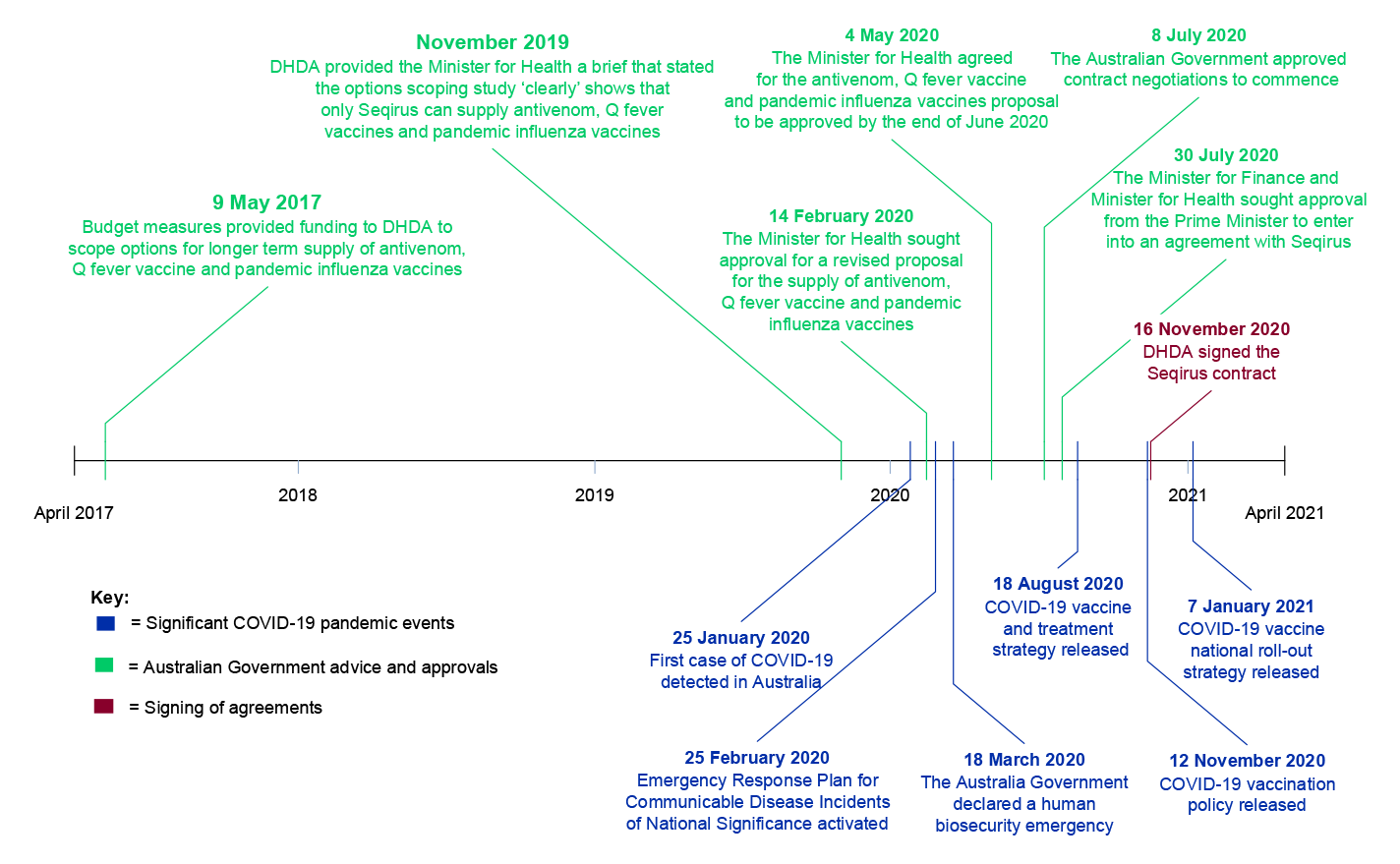
Source: ANAO analysis.
Appendix 4 Timeline of Moderna procurement
Appendix 4 Timeline of Moderna procurement

Source: ANAO analysis.
Appendix 5 Supply options for antivenoms, Q fever vaccine and pandemic influenza vaccine
|
Report |
Purpose / scope |
Conclusions / recommendations |
|
Therapeutic needs and opportunities assessment (draft) Certara, May 2018 |
A due diligence evaluation of 11 therapeutics (snake antivenoms; influenza vaccines; and influenza treatments). The study assessed:
|
Recommendations:
|
|
Biopharmaceutical manufacturing facility study PharmOut, August 2018 |
The study assessed different types of manufacturing methods, including:
|
The study recommended a ‘multipurpose facility with separate dedicated manufacturing areas for each [product], sharing common infrastructure’ and the use of cells to produce influenza vaccines (the existing facility used eggs to produce influenza vaccines, see paragraph 1.6). Costing for the project was $506 million with an accuracy of ±30 to 50 per cent. |
|
Evidence base to inform policy development regarding future long-term supply arrangements for pandemic influenza vaccine (draft) Biointelect and the University of Melbourne, September 2018 |
The study sought to scope viable pandemic influenza vaccine supply arrangements for future pandemics, including risks and benefits for each supply arrangement. The study performed three investigations:
|
The study concluded that:
|
|
Supply options and business advisory project (draft) EY, December 2018 |
The project sought to identify methods to ensure the ongoing supply of antivenoms, Q fever vaccine and pandemic influenza vaccines. The project involved consultation with 27 companies, researchers and institutions. Fourteen options were identified and costed. The 14 options were shortlisted into five viable options.
The review was informed by qualitative analysis of risks and benefits, quantitative analysis of costs and benefits, regulatory context, contractual arrangements, and other research, analysis and modelling. |
The study concluded that:
|
|
Supply options and business advisory project (draft update) EY, July 2019 |
The five options were re-analysed. The two ‘status quo’ options 1 and 2 were based on revised information from Seqirus (see paragraph 2.37), which EY considered to materially impact the costs, risks and economic impacts of options 1 and 2.There were no changes to the government operator and market solution options 3, 4 and 5. |
The updated financial review concluded that:
The review stated that option 4:
|
Source: ANAO analysis.
Footnotes
1 The Prime Minister, Minister for Industry, Science and Technology and Minister for Health, ‘$1 billion manufacturing agreement secures Australia’s national health security’, media release, 16 November 2020, available from https://www.minister.industry.gov.au/ministers/karenandrews/media-releases/1-billion-manufacturing-agreement-secures-australias-national-health-security [accessed 1 July 2025].
2 The Prime Minister, Minister for Industry, Energy and Emissions Reduction, Minister for Health and Aged Care, and Minister for Finance Sector, ‘Partnership secures Australian-made mRNA vaccines’, media release, 24 March 2022, available from https://www.minister.industry.gov.au/ministers/taylor/media-releases/partnership-secures-australian-made-mrna-vaccines [accessed 1 July 2025].
3 The Department of Finance updated the Commonwealth Procurement Rules four times over the course of the procurements audited: in April 2019, December 2020, July 2022 and July 2024. The relevant version was used against each stage of the procurement during this audit as indicated in footnotes.
4 Department of Health, Disability and Ageing, National Medical Stockpile [Internet], DHDA, available from https://www.health.gov.au/our-work/national-medical-stockpile [accessed 13 August 2025].
5 Department of Finance, Buy Australian Plan, Finance, Canberra, 2025, available from https://www.finance.gov.au/business/buyaustralianplan [accessed 1 July 2025].
6 Available from https://www.legislation.gov.au/C2024A00119/asmade/text [accessed 1 July 2025].
7 World Health Organization, Global pandemic influenza action plan to increase vaccine supply, WHO, Geneva, 2006, Abstract, available from https://iris.who.int/bitstream/handle/10665/69388/WHO_IVB_06.13_eng.pdf [accessed 1 July 2025].
8 The Prime Minister, Minister for Industry, Science and Technology, and Minister for Health, ‘$1 billion manufacturing agreement secures Australia’s national health security’, media release, 16 November 2020, available from https://www.minister.industry.gov.au/ministers/karenandrews/media-releases/1-billion-manufacturing-agreement-secures-australias-national-health-security [accessed 1 July 2025]
9 In September 2023 the Prime Minister announced an inquiry into Australia’s response to the COVID-19 pandemic. The COVID-19 Response Inquiry sought to review the Australian Government’s ‘response to the COVID-19 pandemic and make recommendations to improve response measures in the event of future pandemics.’ Terms of reference for the inquiry are available from https://www.pmc.gov.au/resources/commonwealth-government-covid-19-response-inquiry-terms-reference [accessed 1 July 2025].
10 Commonwealth Government, COVID-19 Response Inquiry Report, Commonwealth of Australia, Canberra, 2024, p. 266, available from https://www.pmc.gov.au/resources/covid-19-response-inquiry-report [accessed 1 July 2025].
11 AD Mitchell, ‘The Geography of Health: Onshoring Pharmaceutical Manufacturing to Address Supply Chain Challenges’, World Trade Review, 2024, available from https://www.cambridge.org/core/journals/world-trade-review/article/geography-of-health-onshoring-pharmaceutical-manufacturing-to-address-supply-chain-challenges/120B2E49D4D0CA475F00944F9ACE6172 [accessed 1 July 2025].
12 The Prime Minister, Minister for Industry, Energy and Emissions Reduction, Minister for Health and Aged Care, and Minister for Finance, ‘Partnership secures Australian-made mRNA vaccines’, media release, 24 March 2022, available from https://www.minister.industry.gov.au/ministers/taylor/media-releases/partnership-secures-australian-made-mrna-vaccines [accessed 1 July 2025].
13 The Prime Minister, Minister for Industry, Science and Technology, and Minister for Health, ‘$1 billion manufacturing agreement secures Australia’s national health security’, media release, 16 November 2020, available from https://www.minister.industry.gov.au/ministers/karenandrews/media-releases/1-billion-manufacturing-agreement-secures-australias-national-health-security [accessed 1 July 2025].
14 Except in New Zealand and Antarctica. SP Salifu, AA Bukari, D Frangoulidis, and N Wheelhouse, ‘Current perspectives on the transmission of Q fever: Highlighting the need for a systematic molecular approach for a neglected disease in Africa’, Acta Tropica, 2019, available from https://www.sciencedirect.com/science/article/abs/pii/S0001706X19300889 [accessed 1 July 2025].
15 An Australia Government vaccination program decreased the number of reported Q fever cases by more than 50 per cent between 2001 and 2006. Department of Health, Disability and Ageing, Australian Immunisation Handbook: Q fever, DHDA, Canberra, 2023, see ‘Q fever in Australia’ section available from https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/q-fever [accessed 1 July 2025].
16 President’s Council of Advisors on Science and Technology, Report to the President on reengineering the influenza vaccine production enterprise to meet the challenges of pandemic influenza, Executive Office of the President, USA, August 2010, pp. 12, 54 and 55, available from https://digitalcollections.rice.edu/Documents/Detail/report-to-the-president-on-reengineering-the-infleunza-vaccine-production-enterprise-to-meet-the-challenges-of-pandemic-influenza/266409 [accessed 1 July 2025].
17 AusTender contract notice CN3283185.
18 The Prime Minister, Minister for Industry, Science and Technology, and Minister for Health, $1 billion manufacturing agreement secures Australia’s national health security, Canberra, media release, November 2020, available from https://www.minister.industry.gov.au/ministers/karenandrews/media-releases/1-billion-manufacturing-agreement-secures-australias-national-health-security [accessed 1 July 2025].
19 AusTender contract notice CN3736192.
20 Statistics available from https://covidlive.com.au/archive/20250412 [accessed 5 July 2025]. The Australian Institute of Health and Welfare (AIHW), COVID-19, AIHW, Canberra, 2024, available from https://www.aihw.gov.au/reports/australias-health/covid-19 [accessed 1 July 2025].
21 Australian Government, COVID-19 Response Inquiry Report, Canberra, 2024, p. 50, available from https://www.pmc.gov.au/resources/covid-19-response-inquiry-report [accessed 1 July 2025]. See also: Auditor-General Report No. 3 2022–23, Australia’s COVID-19 Vaccine Rollout, ANAO, Canberra, 2022, available from https://www.anao.gov.au/work/performance-audit/australia-covid-19-vaccine-rollout [accessed 23 July 2025].
22 None of these advance purchasing agreements were published on AusTender.
23 The COVAX Facility managed the end-to-end global procurement and delivery of COVID-19 vaccines. It was a mechanism for high- and upper middle-income countries to gain access to a portfolio of vaccines. In Australia, the COVAX Facility supplied Pfizer vaccines. More information is available from https://www.gavi.org/covax-facility [accessed 1 July 2025].
24 The University of Queensland vaccine did not proceed past clinical trials in December 2020.
25 Four other types of COVID-19 vaccines were in development in 2020 but did not proceed past clinical trials.
26 Mayo Clinic, Different types of COVID-19 vaccines: How they work, Mayo Clinic, 2025, available from https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/different-types-of-covid-19-vaccines/art-20506465 [accessed 1 July 2025].
27 ATAGI advises the Minister for Health and Ageing on the administration of vaccines in Australia. See https://www.health.gov.au/committees-and-groups/atagi [accessed 1 July 2025].
28 Department of Health, Disability and Ageing, ATAGI statement on revised recommendations on the use of COVID-19 Vaccine AstraZeneca, Health, Canberra, 17 June 2021, available from https://www.health.gov.au/news/atagi-statement-on-revised-recommendations-on-the-use-of-covid-19-vaccine-astrazeneca-17-june-2021 [accessed 1 July 2025].
29 Australian Government, COVID-19 Response Inquiry Report, Commonwealth of Australia, Canberra, 2024, p. 264, available from https://www.pmc.gov.au/resources/covid-19-response-inquiry-report [accessed 1 July 2025].
30 The contract was not published on AusTender. When the Commonwealth Procurement Rules are set aside under paragraph 2.6, the requirement, under paragraphs 7.18 to 7.20, to report contracts on AusTender does not apply.
31 The WHO declared the COVID-19 outbreak a public health emergency of international concern on 30 January 2020. Moderna developed the first batch of candidate COVID-19 mRNA vaccine for testing on 7 February 2020. The WHO declared the outbreak to be a pandemic on 11 March 2020.
32 Department of Industry, Science and Resources, 2023-24 Annual Report, DISR, Canberra, 2024, p. 2, available from https://www.industry.gov.au/sites/default/files/2024-10/disr-annual-report-2023-24.pdf [accessed 13 August 2025].
33 The Department of Finance updated the Commonwealth Procurement Rules four times over the course of the procurements: in April 2019, December 2020, July 2022 and July 2024.
34 Department of Health, Disability and Ageing, National Medical Stockpile [Internet], DHDA, available from https://www.health.gov.au/our-work/national-medical-stockpile [accessed 13 August 2025].
35 Department of Finance, Buy Australian Plan, Finance, Canberra, 2025, available from https://www.finance.gov.au/business/buyaustralianplan [accessed 1 July 2025].
36 Available from https://www.legislation.gov.au/C2024A00119/asmade/text [accessed 1 July 2025].
37 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2024, paragraphs 4.2 and 4.4, available from https://www.finance.gov.au/sites/default/files/2024-06/Commonwealth_Procurement_Rules-1-July-2024.pdf [accessed 17 April 2025]. The Department of Finance updated the Commonwealth Procurement Rules four times over the course of the procurements: in April 2019, December 2020, July 2022 and July 2024. This and the other components of the CPRs described in this paragraph were materially unchanged.
38 ibid., paragraph. 4.1.
39 ibid., paragraph. 5.1.
40 ibid., paragraph. 4.5.
41 ibid., paragraph. 6.7.
42 ibid., paragraphs. 7.2 and 7.3.
43 Department of Finance, Contract Management Guide, Finance, Canberra, 2020, p. 2, 2023 version available from https://www.finance.gov.au/sites/default/files/2023-07/australian-government-contract-management-guide-july-2023.pdf [accessed 1 July 2025].
44 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, para. 4.2.
45 AusTender contract notice CN2849352 valued at $983,970.
46 DHDA was unable to locate and provide to the ANAO the final reports for the Certara, Biointelect and EY studies.
47 AusTender contract notice CN3486831 valued at $731,376.
48 AusTender contract notice CN3513835 valued at $761,156.
49 AusTender contract notice CN3518445 valued at $212,000.
50 The contract was not reported on AusTender. The original contract value was $1,163,277, which was varied to $1,302,702.
51 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph. 4.2.
52 ibid., p. 30.
53 Department of Finance, Grants, Procurements and other financial arrangement (RMG 411), Finance, Canberra, 2018, 2024 version available from https://www.finance.gov.au/publications/resource-management-guides/grants-procurements-and-other-financial-arrangements-rmg-411#audience [accessed 1 July 2025]. A grant is defined as ‘providing financial assistance with or without conditions’, while a procurement is defined as ‘acquisition of goods and services for the Commonwealth’s or a third party’s use’.
54 DHDA classifies payments under the contract with Seqirus as grant expenditure for financial accounting purposes. In 2020–21 an initial prepayment of $110 million was recorded in DHDA’s administered financial statements. As the payment constituted a grant expense when incurred, a prior period adjustment was subsequently made in the financial statements to reflect the correct accounting treatment.
55 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2024, paragraph. 4.1. This requirement exists in previous versions of the CPRs.
56 Department of Finance, Commonwealth Risk Management Policy, Finance, Canberra, 2014, 2022 version available from https://www.finance.gov.au/government/comcover/risk-services/management/commonwealth-risk-management-policy [accessed 1 July 2025].
Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraphs 4.4 and 8.2.
57 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 9.2. This requirement exists in previous versions of the CPRs.
58 ibid., paragraph 4.7. The 2024 CPRs require entities to consider economic benefits to the Australian economy for procurements over $1 million.
59 ibid., paragraph 9.10.
60 ibid., paragraph 9.7.
61 AusTender contract notice CN3530692 valued at $252,730.50.
62 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 10.3.d states:
when the goods and services can be supplied only by a particular business and there is no reasonable alternative or substitute for one of the following reasons: …
ii. to protect patents, copyrights, or other exclusive rights, or proprietary information, or
iii. due to an absence of competition for technical reasons …
63 A heads of agreement is a pre-contractual document which outlines the proposed terms of a contract. It is non-binding.
64 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 10.5.
65 ibid., paragraph 7.12.
66 Department of Finance, Commonwealth Risk Management Policy, Finance, Canberra, 2014, 2022 version available from https://www.finance.gov.au/government/comcover/risk-services/management/commonwealth-risk-management-policy [accessed 1 July 2025].
Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraphs 4.4 and 8.2.
67 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 4.5.
68 The contract was not reported on AusTender. The contract value was $660,000.
69 Seqirus advised the ANAO in September 2025 that some confidential information, including pricing for other governments, was not able to be shared with DHDA.
70 The Infrastructure and Project Financing Agency was established on 1 July 2017 as an independent executive agency under the Public Service Act 1999 to support the Australian Government ‘in making commercially astute decisions on nationally significant infrastructure projects and programs’. See https://www.transparency.gov.au/portfolio-entities-companies/infrastructure-transport-cities-and-regional-development/infrastructure-and-project-financing-agency [accessed 1 July 2025].
71 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 8.2.
72 AusTender contract number CN3698191 valued at $25,020, which was varied once to $214,470.
73 The role of the steering committee included: providing strategic advice and direction; ensuring transparency of funding from Seqirus; maximising value for money; approving negotiation strategies and governance; and briefing ministers. The committee comprised secretaries and deputy secretaries from DHDA and the Department of Finance. It was to meet fortnightly.
74 The working group’s roles included: developing negotiation strategies; providing the steering committee with advice and support; reviewing materials from advisers; and secretariat services. The group comprised first assistant secretaries, assistant secretaries and directors from DHDA and the Department of Finance, and commercial, legal and probity advisers.
75 AusTender contract number CN3707154 valued at $440,000.
76 AusTender contract number CN3710827 valued at $245,000, which was varied once to $389,900.
77 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraphs 7.18 and 9.11.
78 Australian Government Department of Finance, Ethics and Probity in Procurement, Finance, Canberra, 2020, 2021 version available from https://www.finance.gov.au/government/procurement/buying-australian-government/ethics-and-probity-procurement [accessed 1 July 2025].
79 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 6.6.
80 DHDA advised the ANAO in February 2025 that DHDA employees now complete a conflict-of-interest declaration for every new or varied procurement.
81 There were two declared conflicts of interest involving family members with shares in CSL, which were managed from that date.
82 Thirteen of the 14 SES officials the ANAO identified to be involved in the procurement and contract management completed general annual material interest declarations in accordance with Australian Government policy. No declarations relevant to the procurement or contract management were identified.
83 Australian Public Service Commission (APSC) guidance states that agency heads are required to provide annual declaration of interests to their minister. See Australian Public Service Commission, ‘Declaration of interests’, Integrity, APSC, 7 March 2019, available from https://www.apsc.gov.au/working-aps/integrity/integrity-resources/declaration-interests [accessed 23 July 2025]. No general declaration was provided to the Minister by the DHDA Secretary in 2020, 2021 or 2022. The DHDA Secretary provided a general declaration to the Minister in August 2023, July 2024 and February 2025. DHDA advised the ANAO in September 2025 that in 2025 the Secretary invested in a managed fund that included CSL shares, that when the Secretary discovered the fund included CSL shares he advised the Australian Public Service Commissioner of the conflict and planned divestiture, and that in February 2025 the Secretary sold the shares in CSL.
84 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 6.6.
85 DHDA’s gifts and benefits register has been maintained since 2019. DHDA was unable to provide to the ANAO any registers pre-dating 2019.
86 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 5.4.
87 Department of Finance, Ethics and Probity in Procurement, Finance, Canberra, 2020.
88 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2019, paragraph 2.10 and Department of Finance, Contract Management Guide, Finance, Canberra, 2020, p. 1.
89 Department of Finance, Contract Management Guide, Finance, Canberra, 2020, p. 2.
90 The Contract Management Guide defines strategic contracts as those that are high value and high risk.
91 DHDA advised the ANAO in January 2025 that it could not locate a risk assessment. The ANAO located the risk assessment in DHDA’s record keeping systems.
92 Department of Finance, Contract Management Guide, Finance, Canberra, 2020, pp. 16–17.
93 DHDA advised the ANAO in June 2025 that it was in the process of updating the plan and had instructed KWM to ensure alignment with the 2020 Seqirus contract, the Contract Management Guide, DHDA’s strategic/complex contract management plan template, and to include a transition plan.
94 DHDA advised the ANAO in June 2025 that staff managing the Seqirus procurement had undertaken appropriate training and provided procurement training completion certificates for relevant officials.
95 Department of Finance, Contract Management Guide, Finance, Canberra, 2020, p. 34.
96 The Workplace Gender Equality Act 2012 requires employers with 500 or more employees to: report annually against six gender equality indicators; have a policy for each of the indicators; and select gender equality targets to achieve or improve against. DHDA’s 2020 contract with Seqirus required Seqirus to provide annual letters of compliance to DHDA.
97 Department of Finance, Contract Management Guide, Finance, Canberra, 2020, pp. 29–30.
98 ibid., p. 30.
99 Department of Health, Disability and Ageing, Australian Health Management Plan for Pandemic Influenza, DHDA, Canberra, 2019, available from https://www.health.gov.au/sites/default/files/documents/2022/05/australian-health-management-plan-for-pandemic-influenza-ahmppi.pdf [accessed 1 July 2025].
100 DHDA advised the ANAO in June 2025 that range of government plans were being updated in light of lessons learns from the pandemic, including the National Health Emergency Response Plan.
101 Paragraph 2.6 of the CPRs states that the CPRs do not apply to the extent that an official applies measures determined by their accountable authority to be necessary for the protection of human health.
102 The CPRs state that documentation commensurate with the scale, scope and risk of the procurement must be maintained (Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2020, paragraphs 7.2 and 7.3). The documentation should provide accurate information on the procurement requirements, processes followed, how value for money was considered and achieved, approval and decisions and the basis for the decisions.
103 Public Governance, Performance and Accountability Act 2013 available from https://www.legislation.gov.au/C2013A00123/latest/text [accessed 1 July 2025].
104 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2020, paragraph 4.2.
105 The report was co-written by DISR, DHDA and the Commonwealth Scientific and Industrial Research Organisation (CSIRO).
106 AusTender contract number CN362306 valued at $2,209,900.
107 DISR advised the ANAO in September 2025 that government decisions were informed by the draft advice, with decisions finalised as part of the 2021–22 Budget via correspondence between the Prime Minister, Treasurer and Minister for Finance.
108 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2020, paragraph 4.2.
109 The Minister for Industry, Science and Technology, ‘Budget boost to manufacturing to secure Australia’s recovery’, media release, 11 May 2021, available from https://www.minister.industry.gov.au/ministers/porter/media-releases/budget-boost-manufacturing-secure-australias-recovery [accessed 1 July 2025].
110 The Prime Minister, Minister for Health and Aged Care and Minister for Industry, Science and Technology, ‘Australia secures Moderna vaccines’, media release, 13 May 2021, available from https://www.minister.industry.gov.au/ministers/porter/media-releases/australia-secures-moderna-vaccines [accessed 11 August 2025].
111 Department of Finance, Grants, Procurements and other financial arrangement (RMG 411), Finance, Canberra, 2018, 2024 version available from https://www.finance.gov.au/publications/resource-management-guides/grants-procurements-and-other-financial-arrangements-rmg-411#audience [accessed 1 July 2025].
112 Department of Finance, Commonwealth Grants Rules and Principles, Finance, Canberra, 2024, available from https://www.legislation.gov.au/F2024L00854/latest/text [accessed 1 July 2025].
113 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2020, paragraph 4.1.
114 Department of Industry, Science and Resources, Approach to Market: proposals to establish an onshore mRNA manufacturing capability, DISR, Canberra, 2021, p. 3, available from https://www.industry.gov.au/sites/default/files/2021-05/approach-to-market-proposals-to-establish-an-onshore-mrna-manufacturing-capability.pdf [accessed 1 July 2025].
115 Department of Finance, Commonwealth Risk Management Policy, Finance, Canberra, 2014, 2022 version available from https://www.finance.gov.au/government/comcover/risk-services/management/commonwealth-risk-management-policy [accessed 1 July 2025]. Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2020, paragraph 4.4. and 8.2.
116 Department of Industry, Science and Resources, Approach to Market: proposals to establish an onshore mRNA manufacturing capability, DISR, Canberra, 2021, p. 3, available from https://www.industry.gov.au/sites/default/files/2021-05/approach-to-market-proposals-to-establish-an-onshore-mrna-manufacturing-capability.pdf [accessed 1 July 2025]. The approach to market request for tender was updated on 24 May 2021 to correct formatting, accessibility and readability.
117 The five dimensions were: product portfolio/pipeline and IP arrangements; manufacturing capabilities and capacity; sustainability, security and flexibility; benefits to the Australian economy; and costs and supports required. Whilst the five dimensions did not cover the approach to market evaluation criteria relating to management of conflicts of interest and confidential information, these were considered in assessments.
118 Department of Finance, Commonwealth Risk Management Policy, Finance, Canberra, 2014, 2022 version available from https://www.finance.gov.au/government/comcover/risk-services/management/commonwealth-risk-management-policy [accessed 1 July 2025]. Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2020, paragraphs 4.4 and 8.2.
119 Department of Finance, Commonwealth Procurement Rules, Finance, Canberra, 2020, paragraph 4.5.
120 AusTender contract number CN3779925 valued at $2,120,250.
121 Engaged probity advisors are discussed later in the chapter.
122 AusTender contract number CN3821232 original value $600,000, which was varied three times to $1,130,000.
123 AusTender contract number for August to December 2021 is CN818217 original value $1,452,000, which was varied twice to $2,568,500. AusTender contract number for January to March 2022 CN3850222 original value $841,5000, which was varied once to $1,683,000.
124 AusTender contract number CN3818062 original value $213,079, which was varied seven times to $2,444,023. KWM’s work under the contract number related solely to the negotiations to establish onshore mRNA manufacturing capability.
125 A break was noted over the Christmas period 2021–22.
126 The National Immunisation Program (NIP) enables eligible people to receive free vaccines to reduce vaccine-preventable diseases. Under the NIP, vaccines require registration with the Therapeutic Goods Administration; a recommendation from the Pharmaceutical Benefits Advisory Committee; the Department of Health, Disability and Ageing and the supplier to agree a vaccine price for the vaccine; approval by the government to list the vaccine on the NIP; legislative amendments; and procurement of the vaccine.
127 The Prime Minister, Minister for Finance, Minister for Health and Aged Care, and Minister for Industry, Energy and Emissions Reduction, ‘mRNA vaccines to be made in Australia’, media release, 14 December 2021, available from https://ministers.finance.gov.au/financeminister/media-release/2021/12/14/mrna-vaccines-be-made-australia [accessed 1 July 2025].
Sarah Martin, ‘Scott Morrison says Omicron won’t “take us back” to Covid restrictions as he announces Moderna deal’, The Guardian, 14 December 2021, available from https://www.theguardian.com/australia-news/2021/dec/14/australia-to-manufacture-mrna-vaccines-under-deal-with-moderna [accessed 1 July 2025].
128 The Prime Minister, Minister for Industry, Energy and Emissions Reduction, Minister for Health and Aged Care, and Minister for Finance Sector, ‘Partnership secures Australian-made mRNA vaccines’, media release, 24 March 2022, available from https://www.minister.industry.gov.au/ministers/taylor/media-releases/partnership-secures-australian-made-mrna-vaccines [accessed 1 July 2025].
129 Department of Finance, Contracting and Report, Finance, Canberra, 2024, see limited tenders at or above the procurement thresholds section, 2024 version available from https://www.finance.gov.au/publications/resource-management-guides/procurement-publishing-and-reporting-obligations-rmg-423/contracting-reporting#-limited-tenders-at-or-above-the-procurement-thresholds- [accessed 23 May 2025].
130 Department of Finance, Resource Management Guide 403: Meeting the Senate Order for Entity Contracts (RMG 403), Finance, Canberra, 2020, 2022 version available from https://www.finance.gov.au/publications/resource-management-guides/meeting-senate-order-entity-contracts-rmg-403 [accessed 6 May 2025].
131 AusTender contract number CN3799470 original value $40,000, which was varied four times to $68,500.
132 AusTender contract number CN3853731 original value $14,456, which was varied once to $36,140.
133 The probity plans were never finalised.
134 DISR advised the ANAO in June 2025 that they are in the process of reviewing their conflict-of-interest policy.
135 The requirement could be waived by the probity advisor or project executive where a previous, relevant conflict of interest declaration could be relied on, or where ‘the completion of the declaration is not practical’.
136 DISR corrected its gifts and benefits register in May 2025. DISR advised the ANAO in June 2025 that it was changing its processes and systems for declaring gifts and benefits.
137 Department of Industry, Science and Resources, Enabling Australia’s onshore mRNA manufacturing capability approach to market, DISR, Canberra, 2021, available from https://www.industry.gov.au/news/enabling-australias-onshore-mrna-manufacturing-capability-approach-market [accessed 1 July 2025].
138 The Contract Management Guide defines strategic contracts as those that are high value and high risk.
139 Department of Finance, Contract Management Guide, Finance, Canberra, 2020, p. 34.