Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Management of Non-Compliance with the Therapeutic Goods Act 1989 for Unapproved Therapeutic Goods

Please direct enquiries through our contact page.
Audit snapshot
Why did we do this audit?
- The Department of Health and Aged Care’s (department’s) monitoring and compliance activities for therapeutic goods are in place to protect Australians from exposure to potentially poor quality, unsafe, or ineffective therapeutic goods, and help to ensure human safety and public health outcomes.
- This audit provides assurance to the Parliament and the Australian community on how effectively the department is managing non-compliance with the Therapeutic Goods Act 1989 and related legislation for the import and supply of unapproved therapeutic goods and the unlawful advertising of therapeutic goods.
Key facts
- The department is responsible for regulating the import, export, manufacture, supply and advertising of therapeutic goods.
- Therapeutic goods include medicines, medical devices, and biologicals.
What did we find?
- The department has been largely effective at managing non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods.
- The department has established a compliance approach for unapproved therapeutic goods that is largely fit for purpose.
- The department’s activities for identifying, preventing and addressing non-compliance with the Therapeutic Goods Act 1989 are implemented largely effectively for the import and supply of unapproved therapeutic goods and the unlawful advertising of therapeutic goods.
What did we recommend?
- There were six recommendations to the department relating to: performance reporting; investigation procedures; investigator qualifications; declarations of interest; complaints handling; and a quality assurance policy.
- The department agreed to all six recommendations.
$1.8m
Total value of infringement notices issued in 2021–22.
8988
Number of compliance actions undertaken in 2021–22 relating to 8625 completed import and supply cases.
1174
Number of online advertisements of therapeutic goods removed in 2021–22.
Summary and recommendations
Background
1. Regulation is an important function of the Australian Government. Effectively administered regulation supports a safe and healthy community; protects people and the environment; and provides a strong setting for business, markets and the economy.1
2. The regulation of therapeutic goods is carried out by the Australian Government’s Department of Health and Aged Care (department). The department uses the ‘Therapeutic Goods Administration’ (TGA) to describe the part of its organisation that regulates therapeutic goods. The TGA is part of the department’s Health Products Regulation Group. The department’s purpose is to ‘lead and shape Australia’s health and aged care system and sporting outcomes through evidence-based policy, well targeted programs, and best practice regulation’.2 In support of this purpose, the department is responsible for regulating the import, export, manufacture, supply and advertising of products defined as therapeutic goods.
3. The Therapeutic Goods Act 1989 (Therapeutic Goods Act) establishes the department’s regulatory powers and underpins the policies, regulations and other legal instruments that support the regulation of therapeutic goods. Under the Therapeutic Goods Act, the department is to:
- assess whether the benefits of a therapeutic good outweigh any risks to health and safety;
- assess the suitability of therapeutic goods for supply, import, and export from Australia;
- regulate manufacturers of therapeutic goods to ensure they meet acceptable standards of manufacturing quality;
- assess the quality and compliance of therapeutic goods on the market; and
- implement a range of regulatory actions that are proportionate to the potential risk arising from non-compliance or emerging safety concerns.
4. Therapeutic goods are divided into four classes, with the two main classes being medical devices and medicines. For the purposes of this audit, the term ‘unapproved therapeutic goods’ includes goods that are being imported, advertised or supplied unlawfully or without authorisation or permission.
5. The department regulates approved and unapproved therapeutic goods by: evaluating and assessing products before they are approved for use in Australia; monitoring products on the Australian market to ensure they comply with regulatory requirements; licensing Australian manufacturers; verifying that overseas manufacturers comply with the same standards as their Australian counterparts; undertaking compliance activities primarily related to the import and advertising of therapeutic goods, but also for the supply and manufacture of unapproved therapeutic goods.
Rationale for undertaking the audit
6. A failure in the department’s compliance activities for therapeutic goods could lead to Australians being exposed to potentially poor quality, unsafe, or ineffective therapeutic goods, with consequences for human safety and public health outcomes. This audit provides assurance to the Parliament on how effectively the department is managing non-compliance with the Therapeutic Goods Act 1989 and related legislation for unapproved therapeutic goods.
Audit objective and criteria
7. The objective of the audit was to assess the effectiveness of the department’s management of non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods.
8. To form a conclusion against the audit objective, the ANAO adopted the following high-level criteria.
- Has the department established a fit-for-purpose compliance approach for unapproved therapeutic goods?
- Are the department’s activities for identifying non-compliance for unapproved therapeutic goods implemented effectively?
- Are the department’s activities for preventing and addressing non-compliance for unapproved therapeutic goods undertaken effectively?
Conclusion
9. The Department of Health and Aged Care’s (department’s) management of non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods was largely effective.
10. The department has established a compliance approach for unapproved therapeutic goods that is largely fit for purpose. The approach includes risk-based compliance priorities, but the procedures and arrangements that underpin the strategy are at a low level of maturity. While the department monitors and reports internally on its compliance activities and prepares some performance information, external reporting of performance could be improved.
11. The department’s activities for identifying non-compliance for unapproved therapeutic goods, such as responding to reports of alleged breaches of the Therapeutic Goods Act 1989, have been implemented largely effectively. There are systems and clear workflows for reactively managing reports of non-compliance; however, there are deficiencies in recordkeeping. In 2021–22, the department started undertaking more proactive activities for identifying non-compliance, particularly by engaging with digital platforms for the removal of advertisements of therapeutic goods, which has resulted in more unlawful advertisements removed from digital platforms.
12. The department’s activities for preventing and addressing non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods are implemented largely effectively. Education and stakeholder engagement activities are implemented effectively and the conduct of compliance responses and investigations are undertaken largely effectively. However, the investigation framework is not fully fit for purpose and the conduct of investigations did not fully align with the Australian Government Investigation Standards 2011.
Supporting findings
Compliance approach
13. The department has established a risk-based strategic approach to the management of non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods. However, most of the compliance plans and procedures that underpin this strategic approach are out of date or in draft form. (See paragraphs 2.2 to 2.20.)
14. The department monitors its compliance activities and there is regular internal reporting about those activities. The department also publishes some performance information about its compliance activities for therapeutic goods in annual reports and on its website. However, there is no reporting against established targets and there is little external information on the performance of the department in achieving the expected outcomes of its compliance approach for therapeutic goods. (See paragraphs 2.21 to 2.38)
Identifying non-compliance
15. The department has effectively implemented procedures for recording and triaging reports of non-compliance with the Therapeutic Goods Act 1989. A clear workflow has been established, although recordkeeping of triage outcomes is poor. An increase in reports of non-compliant imports related to the COVID-19 pandemic resulted in a backlog of reports in early 2022, which had been reduced by June 2022. (See paragraphs 3.3 to 3.18)
16. To identify non-compliance related to the import and supply of therapeutic goods, the department gathers and analyses intelligence and engages with other government entities. The department also undertakes proactive activities for identifying advertising non-compliance, with a focus on monitoring and engagement with digital platforms. These activities have been implemented effectively. The department advised that, in 2022–23, these activities resulted in about 14,000 unlawful advertisements being removed from digital platforms. (See paragraphs 3.19 to 3.31)
Preventing and addressing non-compliance
17. In 2021, the department established an education strategy and education plan for advertising compliance related to therapeutic goods. The department updated its education plan, but not its education strategy, in 2023 to also include import and supply compliance. The updated education plan aligns with the broader compliance priorities. The department has effectively implemented the education strategy and plan and engaged with stakeholders. (See paragraphs 4.3 to 4.19)
18. The department’s investigation policies and procedures for the regulation of therapeutic goods are not mature and do not fully comply with the Australian Government Investigations Standards. Investigator qualifications are not sufficiently monitored, processes for declaring conflicts of interest and complaints handling are not fit-for-purpose, and a quality assurance process has not been established. (See paragraphs 4.20 to 4.50)
19. The department’s compliance investigations and activities for the import and supply of unapproved therapeutic goods, and for the advertising of therapeutic goods, are consistent with the Australian Government Investigations Standards and are undertaken effectively, except for a lack of investigation planning for serious non-compliance cases and supervisor review. (See paragraphs 4.51 to 4.79)
Recommendations
Recommendation no. 1
Paragraph 2.35
The Department of Health and Aged Care review its annually reported performance information for its regulation of therapeutic goods to ensure the information is appropriate and covers the significant components of its key activities.
Department of Health and Aged Care response: Agreed.
Recommendation no. 2
Paragraph 4.27
The Department of Health and Aged Care:
- finalise its investigation procedures for the regulation of therapeutic goods and ensure these procedures align with requirements of the Australian Government Investigations Standard 2022; and
- establish an internal control for the regular review and update of these investigation procedures.
Department of Health and Aged Care response: Agreed.
Recommendation no. 3
Paragraph 4.32
The Department of Health and Aged Care:
- ensure that investigators maintain a minimum level of investigator qualification, as required by the Australian Government Investigations Standard 2022; and
- keep appropriate records of investigator qualifications.
Department of Health and Aged Care response: Agreed.
Recommendation no. 4
Paragraph 4.38
The Department of Health and Aged Care:
- establish an internal control to ensure that officials involved in investigations and compliance activities make and manage declarations of interest; and
- keep appropriate records of declarations of interest.
Department of Health and Aged Care response: Agreed.
Recommendation no. 5
Paragraph 4.46
The Department of Health and Aged Care establish:
- clear complaint handling channels that are accessible and easy to use; and
- a system for end-to-end complaint management and reporting.
Department of Health and Aged Care response: Agreed.
Recommendation no. 6
Paragraph 4.49
The Department of Health and Aged Care develop an Investigations Quality Assurance Policy, as required by the Australian Government Investigations Standard 2022.
Department of Health and Aged Care response: Agreed.
Summary of entity response
20. The proposed audit report was provided to the department. The summary response from the department is provided below. The full response from the department is included at Appendix 1. The improvements observed by the ANAO during the course of this audit are at Appendix 2.
The Department of Health and Aged Care (the department) welcomes the findings in the report and agrees with the recommendations directed to the department.
It was pleasing to note the department has been largely effective in managing non-compliance with the Therapeutic Goods Act 1989 (the Act) and has established a compliance approach for unapproved therapeutic goods that is largely fit for purpose. To further strengthen these arrangements, the department is committed to implementation of the Australian National Audit Office recommendations from this report and has already taken steps to address the issues identified.
The department notes that the audit has identified specific areas for further focus, including performance reporting, investigation procedures, the management of declarations of interest and investigator qualifications, complaints handling, and the establishment of a quality assurance policy.
To address these findings, and to align with the revised Australian Government Investigations Standard 2022, the department has commenced several business improvement initiatives. These activities underpin the compliance work of the Therapeutic Goods Administration and will improve the governance arrangements for managing non-compliance with the Act.
Key messages from this audit for all Australian Government entities
Below is a summary of key messages, including instances of good practice, which have been identified in this audit and may be relevant for the operations of other Australian Government entities.
Program implementation
Performance and impact measurement
Policy design
1. Background
1.1 Regulation is an important function of the Australian Government. Effectively administered regulation supports a safe and healthy community; protects people and the environment; and provides a strong setting for business, markets and the economy.3
Introduction
1.2 The regulation of therapeutic goods is carried out by the Australian Government’s Department of Health and Aged Care (department). The department’s purpose is to ‘lead and shape Australia’s health and aged care system and sporting outcomes through evidence-based policy, well targeted programs, and best practice regulation’.4 In support of this purpose, the department is responsible for regulating the import, export, manufacture, supply and advertising of products defined as therapeutic goods.
1.3 The Therapeutic Goods Act 1989 (Therapeutic Goods Act) establishes the department’s regulatory powers and underpins the policies, regulations and other legal instruments that support the regulation of therapeutic goods. Consistent with the Therapeutic Goods Act, the department is to:
- assess whether the benefits of a therapeutic good outweigh any risks to health and safety;
- assess the suitability of therapeutic goods for supply, import, and export from Australia;
- regulate manufacturers of therapeutic goods to ensure they meet acceptable standards of manufacturing quality;
- assess the quality and compliance of therapeutic goods on the market; and
- implement a range of regulatory actions that are proportionate to the potential risk arising from non-compliance or emerging safety concerns.
Therapeutic Goods Administration
1.4 The department uses ‘Therapeutic Goods Administration’ (TGA) to describe the part of its organisation that is responsible for regulating therapeutic goods. The TGA had an average staffing level of 683 and operating budget of $207.5 million in 2021–22. The TGA comprises three divisions in the department’s Health Products Regulation Group: Medicines Regulation; Medical Devices and Product Quality; and Regulatory Practice and Support. The Regulatory Practice and Support division had an average staffing level of 117 and an operating budget of $77.5 million in 2021–22. The division includes the:
- Regulatory Compliance Branch — which had an average staffing level of 51 in 2021–22 and is responsible for regulatory compliance activities related to the import and supply of unapproved therapeutic goods and the unlawful advertising of therapeutic goods; and
- Regulatory Engagement Branch — which had an average staffing level of 53 in 2021–22 and is responsible for providing regulatory guidance and education materials, planning and performance reporting, committee support and the management of stakeholder engagement, and the TGA website.5
Types of therapeutic goods
1.5 Therapeutic goods are divided into four classes, with the two main classes being medical devices and medicines.
- Medical devices — these include a wide range of products, such as in-vitro diagnostic devices, medical gloves, bandages, syringes, blood pressure monitors, pacemakers and x-ray equipment. They differ from medicines as they generally have a physical or mechanical effect on the body or are used to measure (or monitor) the body and its functions. Medical devices must be ‘included’ on the Australian Register of Therapeutic Goods (ARTG) before they may be supplied in Australia, unless they are exempt from this requirement.6 There were about 62,000 medical devices on the ARTG as at June 2023.
- Medicines — these include prescription medicines, over-the-counter medicines, vaccines and complementary medicines. Medicines must be ‘registered’ or ‘listed’ on the ARTG before they may be supplied in Australia, unless exempted.7 All prescription medicines and most over-the-counter medicines need to be registered, while most complementary medicines (including vitamins, minerals, and herbal and traditional medicines) need to be listed. Registered medicines are assessed for quality, safety and efficacy by the department. The ingredients in listed medicines are assessed for quality and safety, but not efficacy. As at June 2023, there were about 34,000 medicines on the ARTG.
- Other therapeutic goods — these are primarily disinfectants and may be ‘listed’ or ‘registered’ on the ARTG. As at June 2023, there were 311 entries for ‘other therapeutic goods’ on the ARTG — of these, 305 products (98 per cent) were disinfectants, such as those used to clean hospitals.
- Biologicals — these include: tissue-based products; cell-based products; immunotherapy products containing human cells; and products that comprise or contain live animal cells, tissues or organs. Biological products are ‘included’ on the ARTG. There were 45 entries for biologicals on the ARTG as at June 2023.
1.6 Each ARTG entry belongs to a sponsor that is responsible for applying for and maintaining the ARTG entry (including paying annual charges8) and meeting the requirements of the Therapeutic Goods Act. For medicines and biologicals, each separate and distinct therapeutic good needs to be included as a separate entry on the ARTG. For example, there are 669 entries for ‘paracetamol’ on the ARTG. One sponsor has 92 separate ARTG entries for paracetamol, with the differences between these entries including: dosage form (capsule or tablet); dosage; and package type. Only the sponsor of the specific ARTG entry for a particular therapeutic product can legally import, supply or authorise a third party to manufacture, import or supply those products.
1.7 Medical devices are included on the ARTG as a ‘kind of medical device’ which allows low to medium risk devices to be grouped by defined characteristics under one ARTG entry rather than requiring each product to have its own separate entry. Only the sponsor of the specific ARTG entry for a particular therapeutic product can legally import, supply or authorise a third party to import or supply those products.
1.8 Goods on the ARTG are referred to as ‘approved therapeutic goods’. For the purposes of this audit, the term ‘unapproved therapeutic goods’ includes goods that are being imported, advertised or supplied unlawfully or without authorisation or permission (see Box 1).
|
Box 1: Unapproved therapeutic goods (as defined for the purposes of this audit) |
|
Regulation of therapeutic goods
1.9 The department regulates approved and unapproved therapeutic goods by: evaluating and assessing products before they are approved for use in Australia; monitoring products on the Australian market to ensure they comply with regulatory requirements; licensing Australian manufacturers; verifying that overseas manufacturers comply with the same standards as their Australian counterparts; undertaking compliance activities primarily related to the import and advertising of therapeutic goods, but also for the supply and manufacture of unapproved therapeutic goods. The Australian states and territories also regulate possession and some elements of supply of therapeutic goods within their jurisdictions.
1.10 The Therapeutic Goods Act and supporting regulations set out key measures that are designed to support the management of non-compliance with the Therapeutic Goods Act for both approved and unapproved therapeutic goods. These measures include: a range of investigative powers; enforcement actions (such as infringement notices and enforceable undertakings); and criminal offences and civil penalties that may apply where a person breaches a requirement of the Therapeutic Goods Act or engages in behaviour that poses a risk to public health and safety.
1.11 The department is responsible for activities relating to the offence provisions of the Therapeutic Goods Act, including intelligence gathering, compliance activities and enforcement action in relation to unlawful advertising of therapeutic goods and the import, supply, export and manufacturing of unapproved therapeutic goods.
1.12 Since 1 July 2018, the department has been responsible for managing reports about alleged non-compliant advertising of therapeutic goods. In Australia, advertising for consumer goods (including therapeutic goods) must comply with the Competition and Consumer Act 2010 (which incorporates the Australian Consumer Law). However, additional safeguards are in place because therapeutic goods (such as medicines and medical devices) have risks above and beyond ordinary consumer goods. The Therapeutic Goods Advertising Code (the Code) requires advertising to consumers to be ethical and not misleading or deceptive. Certain therapeutic goods, including prescription medicines, biologicals and many over-the-counter medicines are prohibited from being advertised to the public.
Previous reviews of therapeutic goods regulation
Expert Panel Review of Medicines and Medical Devices Regulation
1.13 The Expert Panel Review of Medicines and Medical Devices Regulation (the MMDR Review) was announced by the Australian Government in 2014 and the report was released in 2015. The objective of the MMDR review was to enhance the regulatory framework for medicines and medical devices so that: Australia continued to be well positioned to respond effectively to global trends in the development, manufacture, marketing and regulation of therapeutic goods; and areas of unnecessary, duplicative or ineffective regulation were removed or streamlined without undermining the safety or quality of therapeutic goods available in Australia. The government supported 56 of the MMDR Review’s 58 recommendations. All seven recommendations related to the regulation of therapeutic goods advertising were accepted.
Review of the Therapeutic Goods Advertising Framework
1.14 The 2020 Review of the Therapeutic Goods Advertising Framework (the Advertising Review) was commissioned by the Australian Government to examine the impact and effectiveness of key changes to the advertising framework made following the MMDR Review. The Advertising Review found:
while good progress has been made implementing the Government’s response to the MMDR Report, the focus has necessarily been tactical given the timeframes for implementation. […] The next phase in achieving further effectiveness and impact from the Therapeutic Good Advertising Framework will depend on a more strategic approach.
1.15 The Advertising Review made 22 recommendations, including that the department should:
- develop and publish compliance priorities, which should be reviewed annually, be supported by stakeholder consultation, be focused on consumer benefit and inform key performance indicators and reporting (Recommendation 5);
- develop an education strategy with clearly defined priorities that are aligned to compliance priorities (Recommendation 11); and
- redevelop a suite of advertising compliance performance measures and indicators which focus on priorities and outcomes rather than processes and deadlines. Once the new performance indicators for advertising compliance management have been developed, the TGA [department] should publicise and report against the measures (Recommendations 14 and 15).
Auditor-General reports
1.16 The ANAO has conducted three performance audits involving the department’s regulation of therapeutic goods since 2011–12.
- Auditor-General Report No. 38 2018–19 Application of cost recovery principles identified that the department had been partially effective in implementing the cost recovery principles of the Australian Government’s cost recovery framework.9
- Auditor-General Report No. 30 2013–14 Administering the Code of Good Manufacturing Practice for Prescription Medicines concluded that the department had been generally effective in applying the Code of Good Manufacturing Practice for prescription medicines manufactured or supplied in Australia.10
- Auditor-General Report No. 3 2011–12 Therapeutic Goods Regulation: Complementary Medicines identified that the department’s administration of the regulatory framework would be improved by, among other things, adopting a risk-based approach to compliance monitoring.11
Rationale for undertaking the audit
1.17 A failure in the department’s compliance activities for therapeutic goods could lead to Australians being exposed to potentially poor quality, unsafe, or ineffective therapeutic goods, with consequences for human safety and public health outcomes. This audit provides assurance to the Parliament on how effectively the department is managing non-compliance with the Therapeutic Goods Act and related legislation for unapproved therapeutic goods.
Audit approach
Audit objective, criteria and scope
1.18 The objective of the audit was to assess the effectiveness of the department’s management of non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods.
1.19 To form a conclusion against the audit objective, the ANAO adopted the following high-level criteria.
- Has the department established a fit-for-purpose compliance approach for unapproved therapeutic goods?
- Are the department’s activities for identifying non-compliance for unapproved therapeutic goods implemented effectively?
- Are the department’s activities for preventing and addressing non-compliance for unapproved therapeutic goods undertaken effectively?
1.20 The audit scope focuses on the department’s activities for managing compliance for the import and supply of unapproved therapeutic goods and the unlawful advertising of therapeutic goods. The audit scope covers July 2021 to April 2023 and includes a period where there was an increase in the importation, supply and unlawful advertising of therapeutic goods related to the COVID-19 pandemic. The department advised the ANAO that this ‘had a major impact on staff resource allocation … with a corresponding impact on governance activities that were under way such as the finalisation of policies, manuals and [standard operating procedures]’.
1.21 The audit scope does not include: approvals of therapeutic goods for inclusion on the ARTG; monitoring of safety, quality and efficacy for goods on the ARTG; medicines or devices that are exempt from inclusion on the ARTG but still otherwise able to be legally advertised or sold to the public; oversight or assessment of manufacturing quality; management of medicine shortages; advertising to healthcare professionals; or the cost recovery process.
Audit methodology
1.22 The audit methodology included:
- visits to departmental offices in Canberra, ACT and meetings with departmental officials;
- review of departmental data, documentation, policies, and procedures;
- examination of representative samples of 140 investigations related to product and import compliance and 131 investigations related to advertising compliance, against requirements of the Australian Government Investigations Standards (AGIS) 201112; and
- examination of a targeted sample of 20 investigations of serious non-compliance, against the requirements of the AGIS 2011.
1.23 The audit was conducted in accordance with ANAO Auditing Standards at a cost to the ANAO of approximately $422,000.
1.24 The team members for this audit were Jennifer Eddie, Elizabeth Robinson, Jake Farquharson and Christine Chalmers.
2. Compliance approach
Areas examined
This chapter examines whether the Department of Health and Aged Care (the department), has established a fit-for-purpose compliance approach for the management of non-compliance for unapproved therapeutic goods.
Conclusion
The department has established a compliance approach for unapproved therapeutic goods that is largely fit for purpose. The approach includes risk-based compliance priorities, but the procedures and arrangements that underpin the strategy are at a low level of maturity. While the department monitors and reports internally on its compliance activities and prepares some performance information, external reporting of performance could be improved.
Areas for improvement
The ANAO made one recommendation aimed at improving performance reporting. The ANAO also identified three opportunities for improvement related to strengthening business and risk planning; finalising compliance plans and standard operating procedures; and reporting against performance measures and targets.
2.1 A compliance approach is a set of plans, policies and procedures that establish how a regulator will manage compliance with regulations. The Australian Government’s Regulator Performance Guide outlines regulator best practice.
- Regulators should manage risks proportionately and maintain essential safeguards while minimising regulatory burden.13 A fit-for-purpose compliance approach is risk-based — assisting regulators to gather and use intelligence, target their efforts at the areas of highest risk of non-compliance and respond in a proportionate way to the harm being managed.
- Regulators should have transparent internal and external accountability processes that foster a culture of continuous improvement.14 A fit-for-purpose compliance approach fosters continuous improvement through the measurement and reporting of regulatory performance, including the achievement of regulatory objectives.15
Is there a risk-based strategic approach to the management of non-compliance for unapproved therapeutic goods?
The department has established a risk-based strategic approach to the management of non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods. However, most of the compliance plans and procedures that underpin this strategic approach are out of date or in draft form.
2.2 The department’s regulatory compliance framework for therapeutic goods, which is shown on the Therapeutic Goods Administration (TGA) website, states that its compliance work is guided by five key principles. The second principle states that its compliance and enforcement actions are responsive to risk (Table 2.1).
Table 2.1: Regulatory compliance framework key principles
|
Key principles |
|
1. We promote high levels of voluntary compliance by effectively engaging with and educating the regulated community, with clear guidance on how to comply. |
|
2. Our compliance and enforcement actions are evidence-based and adjust to respond to the nature and seriousness of the alleged non-compliance, and the potential risk to public health and safety. |
|
3. We work with other government bodies, including health and law enforcement agencies, to share information about therapeutic goods, and work collaboratively with them to perform our monitoring and compliance functions. |
|
4. We are committed to transparency and reporting on compliance action. The [Therapeutic Goods Act] allows the [department] to release therapeutic goods information relating to any decision or action taken under the Therapeutic Goods Act or the regulations. |
|
5. We undertake our compliance activities with integrity, professionalism and with due regard to procedural fairness. |
Source: TGA web page ‘Compliance Management’, available from www.tga.gov.au [accessed 14 April 2023].
2.3 The website outlines the range of tools it employs and the types of activities it undertakes to encourage compliance with the Therapeutic Goods Act 1989 (Therapeutic Goods Act) — with the level of response to be proportionate to the level of risk (Figure 2.1). Key components of a risk-based compliance monitoring approach are assessing risks to compliance and developing a compliance strategy.
Figure 2.1: Compliance and enforcement pyramid
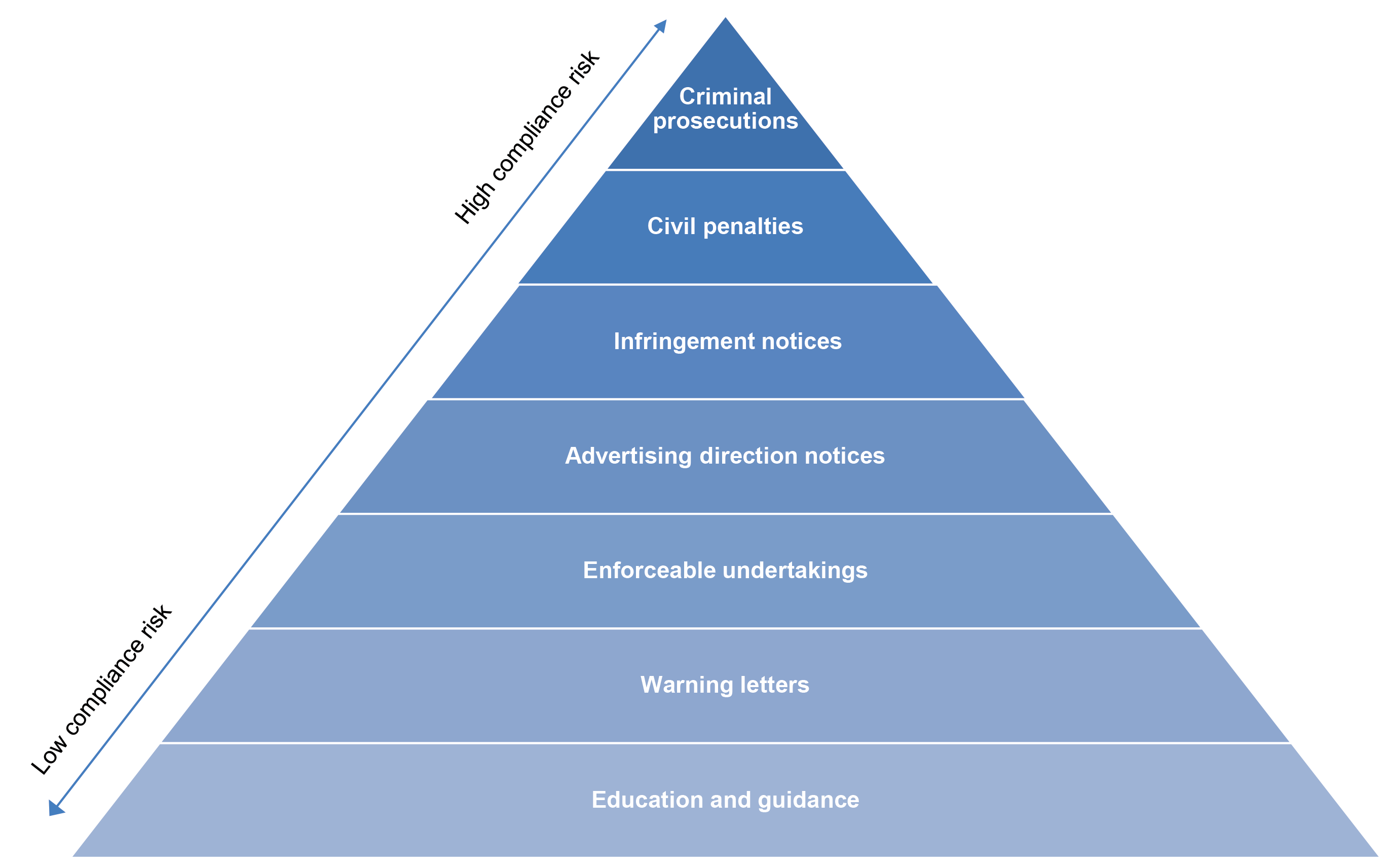
Source: ANAO representation of the department’s compliance and enforcement tools pyramid, from the TGA web page ‘Compliance Management’, available from www.tga.gov.au [accessed 14 April 2023].
Assessing risks to compliance
Departmental risk management framework
2.4 A regulator’s risk management framework should set out risk tolerance levels and inform compliance activity to ensure that risks are maintained within tolerance levels.
2.5 The department reported for 2021–22 that it had worked on various initiatives ‘to improve its maturity in risk management’, including refreshing its risk management policy and updating the department’s enterprise risks and tolerance levels. The department’s August 2022 risk management policy outlines the following ‘enterprise risk tolerance statement’ for regulatory activities:
The Department has a low tolerance for non-compliance with relevant legislation and regulatory activities and requirements.
The Department has a high tolerance for risk-based approaches to regulation and red-tape reduction for business, community organisations and individuals while ensuring the currency of standards for products and services.
2.6 The 2021–22 and 2022–23 business and risk planning documents for the divisions and branches involved in the regulation of therapeutic goods included information on risks and risk mitigation. They did not include risk ratings or provide information on whether they were operating within the department’s risk tolerance levels.
|
Opportunity for improvement |
|
2.7 The Department of Health and Aged Care, in regulating therapeutic goods, could strengthen its business and risk planning to include information on how it will operate within the department’s stated tolerance levels for regulatory activities. |
Regulatory Compliance Risk Committee
2.8 The purpose of the Regulatory Compliance Risk Committee (RCRC) is to promote consistency in the way the department’s Health Products Regulation Group16 considers and manages regulatory and compliance risks. The RCRC is comprised of departmental officers and its functions are to identify and provide advice on emerging issues and provide oversight for the risk management and compliance framework. The RCRC defines risk management in its terms of reference as ‘the set of processes through which [the department] and Health Products Regulation Group identifies, analyses and provides guidance on appropriate responses to issues that may adversely affect the regulation of therapeutic goods and controlled drugs.’ In 2021–22, the RCRC met six times.
Compliance risk assessment
2.9 The department undertook a risk assessment in early 2020–21 to inform the setting of advertising compliance priorities for therapeutic goods in 2020–22. The 2020–22 risk assessment focused on advertising compliance for 17 types of therapeutic goods and issues, assessing the level of risk for each of the therapeutic goods for three factors: threat; vulnerability of the target audience; and harm.
2.10 The department undertook a ‘strategic threat assessment’ to underpin its 2022–23 compliance priorities. The 2022–23 strategic threat assessment rated the risk levels of the 2020–22 compliance priorities and emerging priorities, and forecast whether these risk levels were stable or increasing. This assessment provided information on key drivers behind each risk area and recommendations for updating the compliance priorities.
Developing a compliance strategy
Compliance priorities
2.11 In October 2020, the department established priority areas for advertising compliance with higher risk areas, which had been determined through the 2020–22 risk assessment, given a higher priority level (Table 2.2).
Table 2.2: 2020–22 Advertising compliance priorities
|
Priority level |
Advertising compliance priorities |
|
Priority 1 |
Therapeutic goods associated with COVID-19 |
|
Nicotine vaping productsa |
|
|
Priority 2 |
Stem cell products |
|
Medicinal cannabis |
|
|
Performance and image enhancers |
|
|
Therapeutic goods used in cosmetic industry |
|
|
Hangover cures |
|
|
Weight loss products |
|
|
Priority 3 |
Mental/learning acuity |
|
Bioresonanceb |
|
Note a: Nicotine vaping products were added to the priorities in 2021–22.
Note b: Bioresonance is based on the belief that human beings emit electromagnetic waves, which can only be measured by bioresonance devices. Advertisers claim these devices can measure these waves to detect illness in the human body as well as sending ‘rehabilitated bad’ waves to the patient to alleviate illness.
Source: ANAO analysis of departmental information.
2.12 Nicotine vaping products were added to the compliance priorities following changes to the regulatory environment for these products in 2021–22, as discussed in Case Study 1.
|
Case study 1. Nicotine vaping products |
|
Nicotine vaping products (also known as ‘vapes’, vaping products, electronic cigarettes, or e-cigarettes) contain a solution such as nicotine and are intended to be vaporised or inhaled using a vaping device (such as an e-cigarette or other nicotine delivery system). Using a vaping product is often called ‘vaping.’ Many vaping products contain nicotine, but some do not. Non-nicotine vaping products are not regulated as therapeutic goods. Nicotine vaping products became a top compliance priority in 2021–22. There were increasing concerns about the safety of these products and the rapid growth in use by young people. According to a September 2022 article in the Australian and New Zealand Journal of Public Health:
A 2020 review commissioned by the department concluded that:
The regulatory requirements for nicotine vaping products changed on 1 October 2021, when the entry for nicotine in Schedule 4 to the Standard for the Uniform Scheduling of Medicines and Poisons (Poisons Standard) was amended to capture all nicotine vaping products such as nicotine e-cigarettes, nicotine pods and liquid nicotine as prescription-only medicines.c This means that nicotine vaping products are subject to the regulatory controls for prescription medicines, and the personal importation and domestic purchase of nicotine vaping products requires a doctor’s prescription. In May 2023, the Minister for Health and Aged Care announced further changes to the regulatory environment for nicotine vaping products. This is discussed in Case Study 3 in Chapter 4. |
Note a: Watts, C., Egger, S., Dessaix, A., Brooks, A., Jenkinson, E., Grogan, P. and Freeman, B. (2022), Vaping product access and use among 14–17-year-olds in New South Wales: a cross-sectional study. Australian and New Zealand Journal of Public Health, 46: 814-820, available from https://doi.org/10.1111/1753-6405.13316 [accessed 15 February 2023].
Note b: Banks E, Beckwith K, Joshy G. Summary report on use of e-cigarettes and impact on tobacco smoking uptake and cessation, relevant to the Australian context. Commissioned Report for the Australian Government Department of Health and Aged Care, September 2020, p. 7. Available at http://hdl.handle.net/1885/211618 [accessed 20 February 2023].
Note c: The Poisons Standard is a record of decisions on the classification of medicines and chemicals into Schedules. It is a legislative instrument that includes 10 schedules, including Schedule 4 on prescription only medicines.
2.13 In 2022–23, the department published seven compliance priorities, which included priorities related to the import and supply of unapproved therapeutic goods — in addition to the advertisement of therapeutic goods (Table 2.3). These had been informed by the strategic threat assessment (discussed at paragraph 2.10).
Table 2.3: 2022–23 Compliance priorities for therapeutic goods
|
Key area |
Import, supply and advertisement compliance priorities |
|
COVID-19 related products |
Deter and address the unlawful import, advertising and supply of unapproved therapeutic goods associated with COVID-19 |
|
Nicotine vaping products |
Disrupt and address the unlawful import, advertising and supply of nicotine vaping products |
|
Medicinal cannabis |
Ensure compliance with the requirements of the Therapeutic Goods Act 1989 across the medicinal cannabis industry |
|
Sports supplements and other image enhancing products |
Disrupt and address the unlawful import, manufacture, advertising and supply of unapproved performance and image enhancing therapeutic goods, including sports supplements, with a focus on products containing schedule 4 and 8 poisonsa |
|
Cosmetic products |
Deter and address the unlawful import, advertising and supply of unapproved therapeutic goods used in the beauty and cosmetic dental industry |
|
Advertising |
Address the unlawful use of restricted and prohibited representations in advertisements that have not been approved or permitted, particularly those that target especially vulnerable consumers |
|
Deter and address the unlawful advertising of unapproved therapeutic goods on digital platforms; including for pregnancy and prenatal goods, weight loss products and hangover cures |
|
Note a: The Standard for the Uniform Scheduling of Medicines and Poisons (Poisons Standard) is a record of decisions on the classification of medicines and chemicals into Schedules. It is a legislative instrument that includes 10 schedules, including: Schedule 4 – Prescription only medicines and prescription animal remedies; and Schedule 8 – Controlled drugs.
Source: ANAO representation of the department’s ‘Import, Advertising and Supply Compliance Priorities 2022–23’.
Compliance plans
2.14 Compliance plans cover the scope of the compliance priority or project as well as the activities to be conducted in areas such as education and communications, intelligence, and compliance and enforcement to address non-compliance in particular market segments. As of April 2023, two of six compliance plans had been finalised (Table 2.4).
Table 2.4: Compliance plans for the 2022–23 compliance priorities
|
Priority area |
Compliance plan finalised |
|
COVID-19 related products |
▲ |
|
Nicotine vaping products (finalised March 2021) |
◆ |
|
Medicinal cannabis |
▲ |
|
Sports supplements and other image enhancing products |
▲ |
|
Cosmetic products |
▲ |
|
Advertising on digital platforms (finalised July 2022) |
◆ |
Key: ◆ Compliance plan finalised ▲ Compliance plan not finalised ■ No compliance plan
Source: ANAO analysis of departmental information.
Compliance activity procedures
2.15 In 2021–22, the department created a compliance case workflow to help staff with processing reports of non-compliance for approved and unapproved therapeutic goods. The compliance case workflow was to be comprised of: a high-level workflow showing how teams interact with each other; detailed workflows for stages of the process, such as first and second pass assessments used by a triage team; and standard operating procedures for individual tasks.
2.16 The high-level workflow and a detailed workflow for triage processes, were put into use in 2021–22. An internal report to the RCRC in August 2022 stated that the high-level workflow was ‘the backbone of mapping the compliance process’ for the branch, had helped to ensure consistency and had been a useful tool for training staff.
2.17 As of May 2023, the other planned elements of the compliance case workflow, such as standard operating procedures, were not finalised. The department advised the ANAO that a surge in workload in 2021 and 2022 related to the COVID-19 pandemic and changes to the regulatory framework for nicotine vaping products diverted resources away from business improvement initiatives, including the creation and revision of workflows and standard operating procedures.
2.18 The department started a new governance project in October 2022 to develop, review, consolidate and implement a range of governance documents and measures within the Regulatory Compliance Branch and, where appropriate, the broader Health Products Regulation Group. The project aimed to give the branch a ‘one stop shop’ to support day-to-day duties, including reference materials, standard operating procedures and templates. As part of the governance project, the department has: completed a stocktake of standard operating procedures, templates and policies; reviewed the new Australian Government Investigations Standard (AGIS) 2022 to determine updates needed; updated one standard operating procedure; and prepared an Advertising and Product Investigations Induction Manual for new starters.17
2.19 The stocktake of procedure documents identified 37 standard operating procedures and guides for the Regulatory Compliance Branch. Of these 30 (81 per cent) were in draft or overdue for review. The stocktake also identified 11 process documents in use by the branch and 10 topics where standard operating procedures could be developed but had not been.
|
Opportunity for improvement |
|
2.20 The Department of Health and Aged Care could finalise its compliance plans and standard operating procedures related to the regulation of therapeutic goods. |
Are compliance outcomes being monitored and reported?
The department monitors its compliance activities and there is regular internal reporting about those activities. The department also publishes some performance information about its compliance activities for therapeutic goods in annual reports and on its website. However, there is no reporting against established targets and there is little external information on the performance of the department in achieving the expected outcomes of its compliance approach for therapeutic goods.
2.21 The Australian Government’s Regulator Performance Guide outlines that regulators should have transparent accountability processes, such as internal and external performance monitoring, that foster a culture of continuous improvement.18 The department’s regulatory compliance framework includes the key principle ‘We are committed to transparency and reporting on compliance action’ (Table 2.1).
Internal performance monitoring
2.22 The Regulatory Compliance Branch reports on its activities to the Health Products Regulation Group executive’s quarterly meeting. While the quarterly reports for 2021–22 and 2022–23 did not have performance measures or targets to report against, they did include business information and data, such as:
- project descriptions, such as the governance project;
- the number of advertising enquiries received and finalised;
- education activities;
- compliance actions (including the number of warning letters, advertisements removed from digital platforms, infringement notices, and certificates to facilitate the seizure and destruction of unlawful imports); and
- the number of overdue infringement notices.
2.23 The quarterly reports also included information on assurance activities. For example, the report for quarter four of 2021–22 reported that the branch had assessed 93 closed advertising cases from 2021–22 to determine whether the compliance issues had reoccurred and whether ongoing compliance was being achieved. It reported that for 95 per cent of the cases assessed, the advertiser had remained compliant. The department advised the ANAO that it uses this assurance work to assess its performance for advertising compliance.
2.24 In April 2023, the Regulatory Compliance Branch trialled a new dashboard for inclusion in its quarterly reports. The dashboard included some performance results compared to targets for advertising compliance cases, comprising:
- time to act — action taken within 10 days on high risk and critical cases: 60 per cent (against a target of 100 per cent); and
- time to compliance — finalised within 60 days on high risk and critical cases: 23 per cent (against a target of 90 per cent).
2.25 The department advised the ANAO that it had subsequently removed these two timeliness measures from the dashboard because its case management systems did not contain sufficient data to report against these measures accurately.
2.26 In situations where there were emerging issues and rapid change in the environment, the Regulatory Compliance Branch delivered additional special issue reports to the Health Products Regulation Group executive.
- COVID-19 — weekly reports related to COVID-19 products in 2020 and 2021. These reports contained information such as the number of COVID-19-related: reports of alleged non-compliance with the import, advertising and supply requirements; infringement notices issued; investigations currently active; and investigations closed.
- Nicotine vaping products — since changes were made to the regulation of nicotine vaping products on 1 October 2021, a weekly report has been prepared for the Health Products Regulation Group executive. These reports contain information on the weekly and cumulative numbers of key activities relating to nicotine vaping products, including the number of: investigations commenced and finalised; warning letters and infringement notices issued; nicotine vaping products seized and voluntarily surrendered; and nicotine vaping products tested in departmental laboratories.
External performance reporting
Annual business plans
2.27 The department has been preparing and publishing an annual TGA business plan since 2012–13. The Therapeutic Goods Administration Business Plan 2022–23 introduced 11 key performance indicators (KPIs). The 11 KPIs were linked to four strategic objectives for 2022–23 (Table 2.5). Strategic objective two relates to stakeholder engagement and education activities and strategic objective three relates to compliance activities.
Table 2.5: 2022–23 Business Plan key performance indicators, by strategic objective
|
Strategic objective |
Key performance indicators |
|
1 – Improve public health outcomes through regulation |
1.1 Product approvals and regulatory assessments are delivered in accordance with statutory timeframes and non-statutory targets. |
|
1.2 Provide timely access to innovative therapies and emerging technologies that respond to public health needs. |
|
|
1.3 Propose and support design of regulatory reforms when evidence of value and real benefit is determined, or when risks can be appropriately managed. |
|
|
2 – Actively engage with our stakeholders |
2.1 Respond in a timely manner and effectively to enquiries and be clear about our regulatory decisions. |
|
2.2 Actively communicate and educate stakeholders. |
|
|
2.3 Collaborate with domestic and international health system stakeholders to address regulatory issues and understand the impact of changing policies, practices, and services. |
|
|
3 – Promote compliance with regulatory requirements |
3.1 Data and intelligence are used to identify risks of non-compliance and inform compliance strategy. |
|
3.2 Serious, deliberate, and repeated non-compliance is addressed. |
|
|
3.3 Product safety, quality, efficacy, and performance issues are identified and assessed proportionally with the risk being managed. |
|
|
4 – Innovate and continuously improve |
4.1 Continuously improve services, processes, and systems to ensure they are fit for purpose. |
|
4.2 Promote an impartial, flexible, and innovative workforce. |
|
Source: ANAO analysis of the Therapeutic Goods Administration Business Plan 2022–23.
2.28 The 2022–23 Business Plan stated ‘Reporting on our performance against the TGA Business Plan will primarily include the Department’s Portfolio Budget Statements and Annual Report, and the TGA Performance Report’. KPI 1.1 from the 2022–23 Business Plan is reflected in the department’s 2022–23 Corporate Plan and October 2022–23 Portfolio Budget Statements, which include one measure for the work of the TGA: ‘Percentage of therapeutic goods evaluations that meet statutory timeframes’. The other ten KPIs were not included in the corporate plan or the portfolio budget statements.
Regulatory performance reports
2015–16 to 2020–21
2.29 Under the Australian Government’s Regulator Performance Framework, in force from 1 July 2015 to 30 June 2021, regulators were required to self-assess and report their performance each year against six KPIs and related performance measures. The 2020–21 self-assessment report (which was published on the website) indicated that it had met all six KPIs in relation to its regulation of therapeutic goods (Table 2.6). This included three KPIs related to the department’s compliance work (KPIs 3, 4 and 5). The TGA Industry Forum reviewed the self-assessment report and suggested opportunities for improvement for each of the six KPIs.19
Table 2.6: 2020–21 KPI self-assessment report
|
KPI |
Self-assessment |
|
KPI 1. Regulators do not unnecessarily impede the efficient operation of regulated entities |
Met |
|
KPI 2. Communication with regulated entities is clear, targeted and effective |
Met |
|
KPI 3. Actions undertaken by regulators are proportionate to the regulatory risk being managed |
Met |
|
KPI 4. Compliance and monitoring approaches are streamlined and coordinated |
Met |
|
KPI 5. Regulators are open and transparent in their dealings with regulated entities |
Met |
|
KPI 6. Regulators actively contribute to the continuous improvement of regulatory frameworks |
Met |
Source: ANAO analysis of the TGA Regulator Performance Framework Self-Assessment Report, 2020–21.
2.30 In addition to the self-assessment report, the department published a ‘performance statistics report’ for 2020–21, which included statistics on its regulatory compliance activity for approved and unapproved therapeutic goods. It did not include performance measures or targets.
2021–22 to 2022–23
2.31 The Regulator Performance Guide replaced the Regulator Performance Framework on 1 July 2021. Under the new framework regulators are no longer required to produce a standalone performance report, following a transition year in 2021–22.20 They are instead to report publicly under the Public Governance, Performance and Accountability Act (PGPA Act) through corporate plans and annual reports.
2.32 Although not a requirement, the department published a ‘performance statistics report’ on its regulation of therapeutic goods for 2021–22. This combined the information previously provided as separate self-assessment and performance statistics reports. While the combined report did not contain performance measures, it did contain statistics about regulatory compliance activities for therapeutic goods in 2021–22, such as number of investigations completed and the number of compliance actions taken. This was similar in format to the annual performance statistics reports produced between 2015–16 and 2020–21.21
Performance reporting under the Public Governance, Performance and Accountability Act 2013
2.33 The PGPA Act and the Public Governance, Performance and Accountability Rule 2014 (PGPA Rule) require accountable authorities to measure their entities’ performance.22 The PGPA Rule requires that an entity’s corporate plan includes details of how an entity’s performance will be measured and assessed through performance measures and targets.23 The PGPA Rule sets out the requirements for performance measures, including, among other things, that they: relate directly to one or more of the entity’s purposes or key activities; include measures of the entity’s outputs, efficiency and effectiveness; and provide a basis for an assessment of the entity’s performance over time.24
2.34 The department’s 2021–22 Corporate Plan included four key activities related to the regulation of therapeutic goods under Program 1.8 ‘Health Protection, Emergency Response and Regulation’ (Table 2.7). One of the four key activities (relating to improving consumer access and streamlining regulatory processes) had an associated performance measure, and the activity relating to managing compliance did not have a performance measure. The 2022–23 Corporate Plan also included four key activities for the regulation of therapeutic goods. Of the four key activities, two related to regulatory compliance for unapproved goods, and neither of these two activities were attached to a performance measure.
Table 2.7: Performance information for the regulation of therapeutic goods in Department of Health and Aged Care’s Corporate Plan, 2021–22 and 2022–23
|
Year |
Key Activity (Under Program 1.8) |
Relevant to Managing Compliance? |
Performance Measure |
Target |
|
2021–22 and 2022–23 |
Regulating therapeutic goods, including vaccines, to ensure safety, efficacy, performance, and quality. Promote best practice, monitor compliance, and take appropriate action to address non-compliance. |
Yes |
None |
None |
|
2021–22 and 2022–23 |
Improving access to therapeutic goods for consumers and streamlining regulatory processes for industry. |
No |
Percentage of therapeutic goods evaluations that meet statutory timeframes. |
100% |
|
2021–22 and 2022–23 |
Delivering efficient, best practice therapeutic goods regulatory outcomes through regulatory science excellence, international collaboration and reform in accordance with the Regulatory Science Strategy 2020–2025. |
No |
None |
None |
|
2021–22 |
Undertaking a range of education activities to inform the public and health professionals on reforms to the regulation of prescription opioid medicines. |
No |
None |
None |
|
2022–23 |
Regulating nicotine liquid (vaping) products, including education, compliance, and a 2022 review of this regulation. |
Yes |
None |
None |
Source: ANAO analysis of the Department of Health and Aged Care’s 2021–22 and 2022–23 corporate plans.
Recommendation no.1
2.35 The Department of Health and Aged Care review its annually reported performance information for its regulation of therapeutic goods to ensure the information is appropriate and covers the significant components of its key activities.
Department of Health and Aged Care response: Agreed.
2.36 The Department of Health and Aged Care (the department) will review the performance information provided for the regulation of therapeutic goods against the department’s Performance Reporting Materiality Policy. This will clearly articulate and present the linkages between the purpose of the program, key activities and performance measures.
Advertising compliance reporting
2.37 Since 1 July 2018, the department has been responsible for receiving and acting on reports about the unlawful advertising of therapeutic goods. From 2018–19 to 2021–22, the department published an annual report on the regulation of therapeutic goods advertising. While the annual advertising compliance report contains activity data and information (such as the number of: reports of alleged non-compliance received by compliance priority; cases created; cases closed; and compliance actions taken), it does not contain performance measures with targets. The 2021–22 report also included information on its compliance assurance work. The department reported that it had assessed 263 advertising cases closed in 2021–22 to examine whether compliance was ongoing, and that for 96 per cent of these cases, ongoing compliance was achieved (that is, the advertiser had remained compliant).
|
Opportunity for improvement |
|
2.38 The Department of Health and Aged Care could ensure that public reporting on advertising compliance activities for therapeutic goods includes performance measures and that all measures have targets, where practicable. |
3. Identifying non-compliance
Areas examined
This chapter examines whether the Department of Health and Aged Care’s (department’s) activities for identifying non-compliance with the Therapeutic Goods Act 1989 (Therapeutic Goods Act) for unapproved therapeutic goods have been implemented effectively.
Conclusion
The department’s activities for identifying non-compliance for unapproved therapeutic goods, such as responding to reports of alleged breaches of the Therapeutic Goods Act 1989, have been implemented largely effectively. There are systems and clear workflows for reactively managing reports of non-compliance; however, there are deficiencies in recordkeeping. In 2021–22, the department started undertaking more proactive activities for identifying non-compliance, particularly by engaging with digital platforms for the removal of advertisements of therapeutic goods, which has resulted in more unlawful advertisements removed from digital platforms.
Areas for improvement
The ANAO identified two opportunities for improvement related to improving recordkeeping and finalising the standard operating procedures for monitoring advertisements on digital platforms.
3.1 Effectively detecting non-compliance allows the department to take action to stop the import, supply and advertising of unapproved therapeutic goods in Australia. The Australian Government Investigations Standards (AGIS) 2011 (updated in 2022) established requirements for effective detection of non-compliance.25 The AGIS is intended to articulate Australian Government policy and is the foundational standard for entities conducting investigations relating to the government programs and legislation they administer.
3.2 The department’s regulatory compliance framework for the regulation of therapeutic goods states that it uses a combination of reactive and proactive detection activities to support its compliance activities. It also states that as it cannot pursue all matters that come to its attention, it focuses on those matters that concern public safety, allege serious breaches of the Therapeutic Goods Act and regulations, and involve repeated or wilful non-compliance.
Are reactive activities for identifying non-compliance implemented effectively?
The department has effectively implemented procedures for recording and triaging reports of non-compliance with the Therapeutic Goods Act 1989. A clear workflow has been established, although recordkeeping of triage outcomes is poor. An increase in reports of non-compliant imports related to the COVID-19 pandemic resulted in a backlog of reports in early 2022, which had been reduced by June 2022.
Reports of non-compliance
3.3 In relation to receiving reports of non-compliance, the AGIS 2011 outlined that agencies ‘where appropriate should have an email address, phone number, fax number or online system for receiving information or referrals from the public. These should be published on the agency’s website’.26
3.4 The website has a web page entitled ‘Report a breach’ which includes links to:
- an online form for reporting a perceived breach of the Therapeutic Goods Act, counterfeit products or questionable practices in relation to therapeutic products;
- an online form for reporting non-compliant advertising for a medicine, medical device or any other type of therapeutic good; and
- a ‘Contact us’ web page with contact details, such as telephone numbers, email address, fax number, postal address, social media links and links to further information.
3.5 The department groups non-compliance into two categories: ‘product and import’ non-compliance (including the import, supply, manufacture and export of unapproved therapeutic goods) and advertising non-compliance (which covers both unapproved and approved therapeutic goods).
- Product and import non-compliance — in 2021–22, the department received 9509 reports of alleged non-compliance related to unapproved therapeutic goods. Of these, 97 per cent related to the import of unapproved therapeutic goods. Of the remaining three per cent (268 reports), there were: 261 reports related to supply; four related to manufacturing; and three related to export.
- Advertising non-compliance — in 2021–22, the department received 2991 reports of alleged advertising non-compliance. This comprised 1950 reports related to the 2021–22 compliance priorities and 1041 reports that were not linked to a compliance priority (Table 3.1).
Table 3.1: Reports of advertising non-compliance received in 2021–22
|
Priority level |
2021–22 compliance priority |
Reports receiveda |
|
1 |
COVID-19 |
747 |
|
Nicotine vaping products |
263 |
|
|
2 |
Stem cells |
5 |
|
Medicinal cannabis |
226 |
|
|
Performance and image enhancers |
143 |
|
|
Therapeutic goods used in the cosmetic industry |
211 |
|
|
Hangover cures |
8 |
|
|
Weight loss products |
56 |
|
|
3 |
Mental/learning acuity |
11 |
|
Bioresonance |
280 |
|
|
Subtotal — Reports related to compliance priorities |
1950 |
|
|
Other |
1041 |
|
|
Total |
2991 |
|
Note a: Multiple reports may relate to the same case (for example, the department could receive multiple reports about the same non-compliant advertisement). A report may be recorded as a case ‘for information only’ and closed rather than progressing as a case for action by a compliance response or investigation team.
Source: ANAO analysis of departmental information reported in the Therapeutic Goods Advertising Compliance Annual Report 2021–22. p. 15.
3.6 The department receives reports of non-compliance from a range of sources, such as consumers, government bodies and health practitioners. For reports related to product and import non-compliance in 2021–22, 97 per cent of reports came from the Australian Border Force (ABF), a group within the Department of Home Affairs.27 The remaining three per cent of reports came primarily from the public, state health departments, law enforcement and medical practitioners.
3.7 For reports of alleged advertising non-compliance in 2021–22, 62 per cent (1852 of 2991) came from consumers and 13 per cent (375 of 2991) came from government or statutory bodies (Figure 3.1).
Figure 3.1: Sources of reports of advertising non-compliance, 2021–22
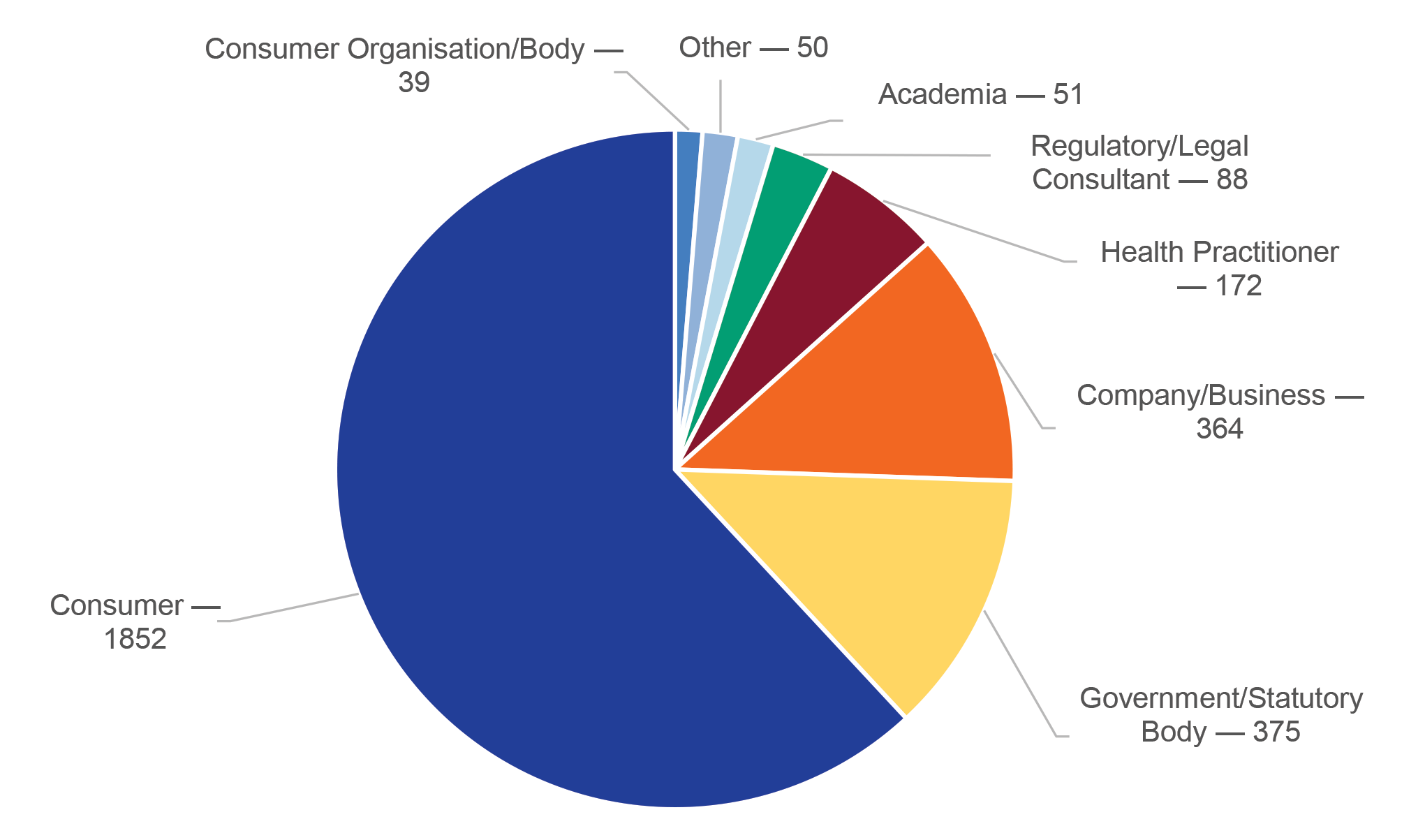
Source: ANAO representation of information from the Therapeutic Goods Advertising Compliance Annual Report 2021–22, p. 14.
System for recording reports of non-compliance
3.8 The AGIS 2011 requires that agencies have ‘an electronic system for recording the receipt of referrals or conduct identified as allegedly, apparently or potentially breaching the law’.28 The department uses two electronic case management systems for recording the receipt of reports of alleged breaches of the Therapeutic Goods Act — one system for import and supply non-compliance and one system for advertising non-compliance.29 The department advised the ANAO that having two systems is inefficient and that it intends to eventually merge the two systems.
Triaging reports of non-compliance
Triage process
3.9 Once a report has been added to one of the two case management systems, it becomes a ‘case’ (even if the case is closed without proceeding to a compliance response or investigation).
3.10 The Regulatory Compliance Branch focuses its resourcing on: cases that relate to a compliance priority; cases that have come from the ABF; and other high-risk cases. The department has established a first and second pass assessment workflow to guide its triage of reports of alleged non-compliance. The first pass assessment involves a series of questions, which result in the case being closed or the case moving to a second-pass assessment, as shown at Figure 3.2.
Figure 3.2: First pass assessment of a report of alleged non-compliance
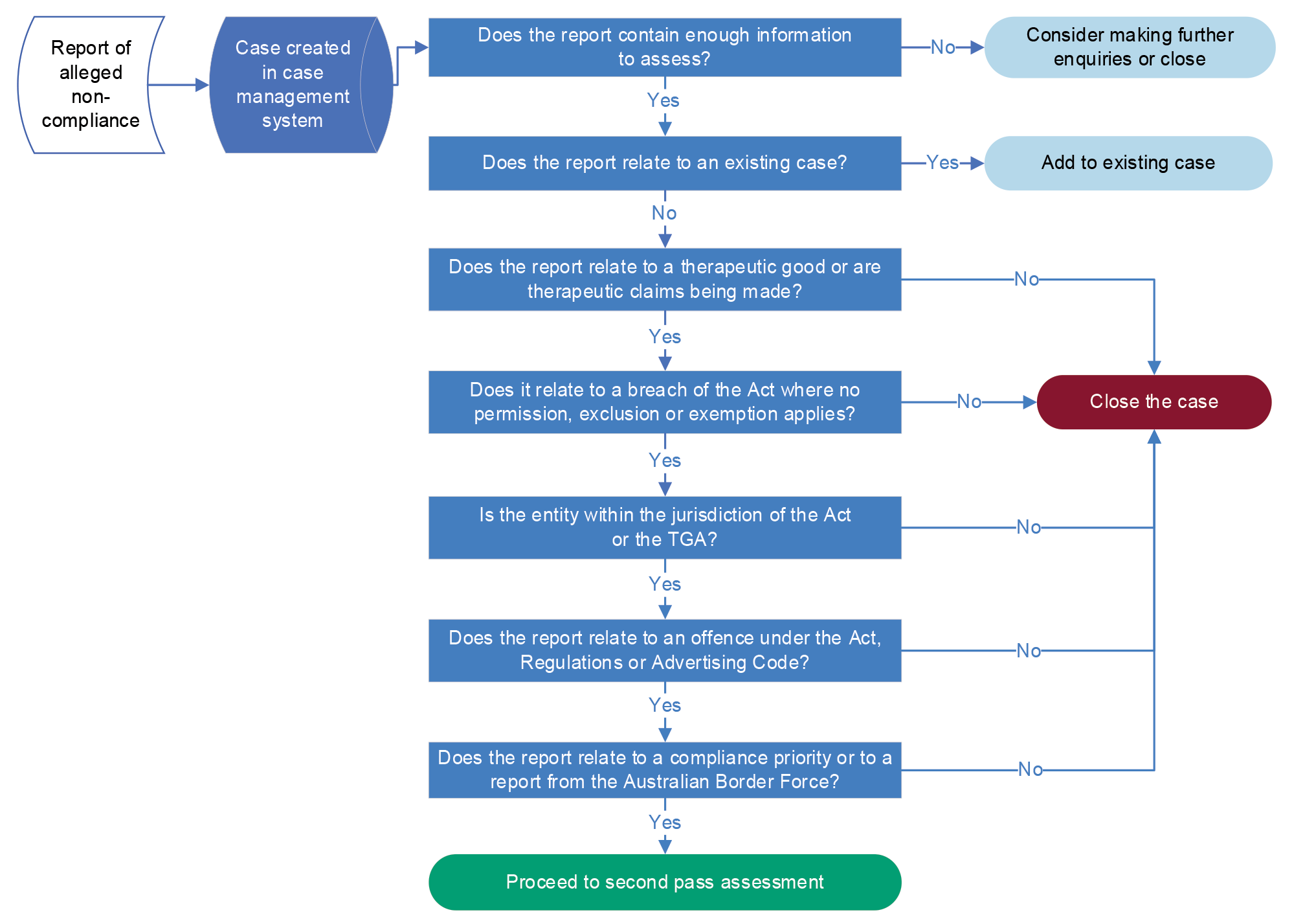
Source: ANAO representation of departmental information.
3.11 For the second pass assessment, the triage team seeks to determine if the entity’s alleged actions constitute serious non-compliance and therefore whether the case should be sent to the serious non-compliance investigation team. The triage team is to refer the case to the serious non-compliance team for investigation if the alleged non-compliance is characterised by: repeated actions of non-compliance; wilful or knowing misconduct; or a threat to the objectives of the regulatory system.30 If the case is not categorised as serious non-compliance, it is referred to the ‘product and import compliance’ team (for import, supply, export and manufacturing matters) or the advertising compliance team.
3.12 The ANAO found that the workflow was generally being followed. For the cases examined, the ANAO found that triage officers sometimes took action (such as issuing a warning to advertisers or requesting the removal of an advertisement on a digital platform). This occurred for 10 of 131 advertising cases sampled by the ANAO (discussed further at paragraph 4.67). The department advised the ANAO that this process is ‘currently under review’.
3.13 The way the department records case outcomes in its case management system makes it difficult to determine the percentage of cases closed at triage. In the case management system used for advertising, 21 different ‘outcomes’ for a case are recorded at case closure by both triage officers and investigators. The different recorded ‘outcomes’ do not provide clear information about the stage of closure or the reasons for closure as many of the 21 ‘outcomes’ are vague or not mutually exclusive. The ANAO also found poor recordkeeping, with 553 (23 per cent) of 2376 advertising cases in 2021–22 having been closed with no record of who closed them. Lack of clarity in case records impedes transparency and accountability, case review, and an understanding of compliance and regulatory trends.
|
Opportunity for improvement |
|
3.14 The Department of Health and Aged Care could strengthen recordkeeping for its case management systems for the regulation of therapeutic goods. |
Triage timeliness
3.15 The department established a performance measure for timeliness of action (creation of a case in the case management system) on advertising reports in 2020 — Time to act: ‘100 per cent in 10 days’ (as discussed at paragraph 2.24). The department advised the ANAO in June 2023 that it has been unable to report accurately against this KPI due to limitations in its IT systems. The ANAO examined a sample of 131 advertising cases (of a population of 2376 cases closed in 2021–22) and found that 54 per cent of cases were created within 10 days of the report of non-compliance being received and 18 per cent took longer than 90 days to action.
3.16 The department does not have a timeliness service standard for product and import cases. The ANAO examined a sample of 140 product and import cases (of a population of 8625 cases closed in 2021–22) and found that 71 per cent had been triaged within 10 days of the report being received (Table 3.2).
Table 3.2: Timeliness of triage for closed cases, 2021–22
|
Timeframe |
Product and importa |
Advertisingb |
|
Action taken within 10 days |
99 (71%) |
70 (54%) |
|
Action taken between 11 and 30 days |
33 (24%) |
13 (10%) |
|
Action taken between 31 and 60 days |
6 (4 %) |
15 (12%) |
|
Action taken between 61 and 90 days |
2 (1%) |
8 (6%) |
|
Action taken after 90 days |
0 (0%) |
24 (18%) |
|
Total |
140 |
130c |
Note a: For product and import cases closed in 2021–22, the timeframes relate to the number of days from the report date until action was taken (such as warning letter issued).
Note b: For advertising cases closed in 2021–22, the timeframes relate to the number of days from the date the advertising non-compliance was reported until case creation.
Note c: The total does not add up to 131 as for one case there was a data issue where the action date was a date before the report received date. Percentages are based on 130 cases.
Source: ANAO analysis of departmental information.
Reports awaiting triage
3.17 In April 2022, the department had a backlog of 2765 reports on import non-compliance from the ABF that were awaiting triage. Each report represented one or more consignments of physical goods held at ABF facilities and awaiting a decision by the department. These reports largely related to COVID-19 medicines and medical devices. The department advised the ANAO:
During the pandemic there was a significant increase in the number of referrals from Australian Border Force of therapeutic goods detected at the border. In the month of February 2022, more referrals were received from [the] ABF than in 2018, 2019 and the first half of 2020 combined. The exponential increase in referrals placed extreme pressure on [branch] resourcing.
3.18 The department managed this largely through a bulk round of 2200 warning letters in June 2022.31 By 7 July 2022, the bulk round was nearing completion, with about 2200 cases closed. The department also increased staffing levels to manage the increased rate of reports from the ABF.
Are proactive activities for identifying non-compliance implemented effectively?
To identify non-compliance related to the import and supply of therapeutic goods, the department gathers and analyses intelligence and engages with other government entities. The department also undertakes proactive activities for identifying advertising non-compliance, with a focus on monitoring and engagement with digital platforms. These activities have been implemented effectively. The department advised that, in 2022–23, these activities resulted in about 14,000 unlawful advertisements being removed from digital platforms.
3.19 Proactive activities for identifying non-compliance include intelligence gathering, engagement with other government entities, and monitoring and engagement with digital platforms.
Intelligence gathering
3.20 The department defines the goal of intelligence work as ‘to collect, analyse and disseminate information to support enhanced decision-making through the identification of trends, challenges, threats and opportunities’.
3.21 The department has allocated one officer to gather and analyse operational information to help identify areas of compliance and enforcement action that would have the largest impact in disrupting non-compliant behaviour related to unapproved therapeutic goods. The intelligence officer prepares operational intelligence reports on compliance topics, such as sports supplements and liquid nicotine, for operational areas. The intelligence officer also responds to specific requests from operational teams for intelligence on particular compliance topics or targets and produces information reports using open-source information on domestic and international activity that may influence trends and behaviour related to therapeutic goods in Australia.
3.22 In addition, in 2021–22, the department engaged Thales Australia Pty Ltd to prepare two open-source intelligence insight briefs on liquid nicotine and sports supplements, under a $28,000 contract. These briefs were completed in November and December 2021.
Engagement with other government entities
3.23 The department has established relationships with other government entities for intelligence sharing and receives intelligence related to non-compliance with the Therapeutic Goods Act from Commonwealth and state government entities, including: the ABF; Australian Competition and Consumer Commission; Australian Transaction Reports and Analysis Centre; Australian Federal Police; and state law enforcement and health departments. The department has also established relationships with international law enforcement agencies, such as INTERPOL, to support international information sharing and cooperation.
3.24 The department also has an ongoing relationship with the ABF in relation to border control of imports of therapeutic goods. The ABF is responsible for monitoring and stopping illicit and unlawful imports at the border, which includes unlawfully imported therapeutic goods. Imported therapeutic goods that have come to the attention of the ABF and are suspected to be an unlawful import are referred to the department. The department is then responsible for assessing these cases to determine if the goods are approved or have an exemption. Most assessments are paper based, but in some cases the department requests a sample of the goods for testing in its laboratories. The department has an agreement with the ABF for the sampling of therapeutic goods for the purposes of enforcement of provisions of the Therapeutic Goods Act. The department also provides the ABF (at least twice each year) with lists of the therapeutic goods and active pharmaceutical ingredients that are its highest priorities.
Monitoring of and engagement with digital platforms
3.25 The department’s regulatory compliance framework includes among its key principles: ‘We work with other government bodies, including health and law enforcement agencies, to share information about therapeutic goods, and work collaboratively with them to perform our monitoring and compliance functions’ (Table 2.1). One of the 2022–23 compliance priorities is also to ‘deter and address the unlawful advertising of unapproved therapeutic goods on digital platforms’. The approach to this includes several proactive elements:
- establishing ongoing relationships with digital platforms;
- providing information and guidance about advertising requirements for therapeutic goods to help digital platforms in strengthening their community guidelines, with the goal of preventing unlawful advertisements from occurring32;
- monitoring of digital platforms for unlawful products; and
- establishing processes to report unlawful advertisements to the relevant platform.
3.26 In 2021–22, the department started undertaking more activities related to advertising therapeutic goods on digital platforms, such as monitoring platforms for non-compliant advertisements and engaging directly with digital platforms to request the removal of content. In 2021–22, the department reported that it was targeting the advertising of nicotine vaping products by social media influencers on Instagram, Facebook, Snapchat and TikTok.33 This involved seeking the cooperation of various social media platforms to remove advertisements that were non-compliant.
3.27 In 2022–23, the department established or renewed relationships with a number of digital platforms. The department advised the ANAO that this involved establishing key contacts within these organisations, providing input into user policies, establishing reporting mechanisms and meeting with these organisations about twice each year (or more often when new issues arose).
3.28 The department advised the ANAO that, between July 2022 and March 2023 it monitored digital platforms for weight loss products, pregnancy and prenatal products, hangover cures and other emerging goods. The department advised that during this nine-month period, it requested the removal of 13,793 advertisements and pieces of content from digital platforms.34 In 2021–22, 1174 advertisements were removed from platforms and 569 advertisements were removed in 2020–21.
3.29 An example of how the department works with digital platforms to address unlawful advertising of therapeutic goods is discussed in Case Study 2.
|
Case study 2. Proactive activities to identify and address unlawful advertisements of Melanotan on digital platforms |
|
Melanotan-I (afamelanotide) is a prescription-only medicine and regulated as an approved therapeutic good in certain circumstances and for specific therapeutic uses. There are no Melanotan-II products listed on the Australian Register of Therapeutic Goods. Melanotan-I and (especially) Melanotan-II are typically promoted as tanning products and may be in the form of pills, creams, injectables and nasal or misting sprays. Misuse of this product can cause serious side effects. It is illegal to advertise prescription-only medicines, such as Melanotan-I, and unapproved therapeutic goods, such as Melanotan-II, to the public. By October 2022, the department had received a number of reports of advertisements of Melanotan on digital platforms. The department conducted searches for Melanotan across digital platforms and identified significant amounts of Melanotan advertising. The department submitted advertisement removal requests to three digital platforms. As a result of these requests, 499 advertisements relating to Melanotan were removed from digital platforms. The department advised the ANAO that a digital platform’s banning of relevant hashtags also had an impact on the non-compliant advertising of Melanotan. In October 2022, the department issued a media release that urged consumers to avoid using products containing Melanotan without advice from a medical professional and warned advertisers, including social media influencers:
|
3.30 The department has prepared standard operating procedures for staff outlining how to proactively scan digital platforms and identify and request the removal of non-compliant advertisements of therapeutic goods. As at March 2023, these procedures were in draft form.
|
Opportunity for improvement |
|
3.31 The Department of Health and Aged Care could finalise its standard operating procedures for monitoring therapeutic goods advertising on digital platforms. |
4. Preventing and addressing non-compliance
Areas examined
This chapter examines whether the Department of Health and Aged Care’s (department’s) activities for preventing and addressing non-compliance with the Therapeutic Goods Act 1989 (Therapeutic Goods Act) for unapproved therapeutic goods have been implemented effectively.
Conclusion
The department’s activities for preventing and addressing non-compliance with the Therapeutic Goods Act 1989 for unapproved therapeutic goods are implemented largely effectively. Education and stakeholder engagement activities are implemented effectively and the conduct of compliance responses and investigations are undertaken largely effectively. However, the investigation framework is not fully fit for purpose and the conduct of investigations did not fully align with the Australian Government Investigation Standards 2011.
Areas for improvement
The ANAO made five recommendations aimed at improving: written investigation procedures; the management of investigator qualifications; management of declarations of interest; complaints handling; and establishing a quality assurance policy. The ANAO also identified three opportunities for improvement related to: the compliance education strategy; investigation plans; and supervisor review.
4.1 A regulator’s education activities and engagement with regulated entities and other stakeholders can make an important contribution to the prevention of non-compliance. The Regulator Performance Guide outlines that: ‘Best practice regulators are transparent, open and responsive to feedback on how they operate, engaging in genuine two-way dialogue with stakeholders and the broader community on their performance’ and that effective stakeholder guidance and information helps ‘regulated entities understand their obligations and responsibilities’ and encourages voluntary compliance.35
4.2 The Therapeutic Goods Act and supporting regulations set out key measures that are designed to support compliance with the Therapeutic Goods Act and its objectives, principally in relation to ensuring the quality and safety of therapeutic goods available in Australia. These measures include: a range of investigative powers; enforcement responses, such as infringement notices and enforceable undertakings; and criminal offences and civil penalties that may apply where a person breaches a requirement of the Therapeutic Goods Act or engages in behaviour that poses a risk to public health and safety (such as, supplying counterfeit therapeutic goods). The department also uses non-statutory compliance responses, such as warning letters, to address non-compliance. The Australian Government Investigations Standards (AGIS) are the minimum standards for government entities conducting investigations relating to the programs and legislation they administer. The AGIS outline standards for a regulator’s investigation system and for all stages of an individual investigation.
Are education and stakeholder engagement activities for preventing non-compliance implemented effectively?
In 2021, the department established an education strategy and education plan for advertising compliance related to therapeutic goods. The department updated its education plan, but not its education strategy, in 2023 to also include import and supply compliance. The updated education plan aligns with the broader compliance priorities. The department has effectively implemented the education strategy and plan and engaged with stakeholders.
4.3 The department’s regulatory compliance framework for therapeutic goods includes the key principle: ‘We promote high levels of voluntary compliance by effectively engaging with and educating the regulated community, with clear guidance on how to comply’ (Table 2.1).
Education strategy and plan
4.4 In July 2021, the department released the Therapeutic Goods Advertising Compliance Education Strategy (Education Strategy), which states:
Our education strategy is to maximise compliance, and minimise inadvertent non-compliance with the advertising rules, for the benefit of Australians, by: providing fit for purpose information and educational resources; leading and participating in education and training opportunities; generating targeted compliance and enforcement-based communications; and providing an efficient enquiry management function.
4.5 The Education Strategy includes six education priorities for advertising compliance (Table 4.1).
Table 4.1: 2021 Education priorities for advertising compliance
|
Priority number |
Education Priority |
|
1 |
Plan for and develop communication and education products as part of the Compliance Plans for the agreed advertising compliance priorities. |
|
2 |
Timely communications on regulatory changes and emerging issues. Examples include fact sheets, targeted information particularly for new sponsors entering the therapeutic goods market, and specific guidance on key issues from time to time. |
|
3 |
Publish information on compliance and enforcement actions for transparency of compliance activities and as a deterrent to non-compliance. |
|
4 |
Maintain and enhance fit for purpose educational resources and participate in training and education opportunities. |
|
5 |
Engage with key stakeholders, including members of the Therapeutic Goods Advertising Consultative Committee (TGACC), as partners in education and communication activities. |
|
6 |
Maintain and enhance an advertising enquiry management function as a key educational tool. |
Source: ANAO analysis of information in the Therapeutic Goods Advertising Compliance Education Strategy, July 2021.
4.6 The Education Strategy does not address education related to the import and supply of therapeutic goods.
|
Opportunity for improvement |
|
4.7 The Department of Health and Aged Care could update its Education Strategy for the regulation of therapeutic goods to include all compliance priorities, including those for import and supply. |
4.8 The department released the first Advertising Compliance Education Plan (Advertising Education Plan) in November 2021 to underpin the Education Strategy. The advertising Education Plan listed education activities and materials against the six education priorities and for five of the ten 2021–22 advertising compliance priorities.36 The Advertising Education Plan also identified education activities related to regulatory changes and emerging issues, beyond the agreed annual compliance priorities. This included producing guidance for changes to the advertising complaints handling framework, medical devices reforms, and changes to the Therapeutic Goods Advertising Code.
4.9 The department released an updated Education Plan in February 2023. The 2023 Education Plan outlined education activities that aligned with each of the 2022–23 import, advertising and supply compliance priorities. The 2023 plan also identified activities relating to regulatory changes and emerging issues, such as medical device reforms and information for advertisers of therapeutic goods.
Education activities
4.10 The department states on the website ‘we recognise education and guidance are key to encouraging and assisting with compliance with Australian regulation’.37 The department publishes information, conducts training, and answers enquiries to assist industry in understanding and meeting the regulatory requirements under the Therapeutic Goods Act.
4.11 The activities outlined in the Advertising Education Plan include: developing communication and education products for advertising compliance priorities; providing webinars on key topics; presenting at external events; providing information to the public and key stakeholders on regulatory changes, emerging issues and compliance and enforcement action; and managing enquiries related to advertising compliance.
4.12 In 2021–22, the department undertook activities for each of its six education priorities (Table 4.2).
Table 4.2: Education activities by education priority, 2021–22
|
Priority number |
Education Priority |
Education activities in 2021–22 |
|
1 and 4 |
Education products, resources and training |
|
|
2 |
Communications on regulatory changes and emerging issues |
|
|
3 |
Information on compliance and enforcement actions |
|
|
5 |
Engagement with key stakeholders |
|
|
6 |
Advertising enquiry management function |
|
Source: ANAO analysis of departmental information.
4.13 As outlined in Table 4.2, the department engages with stakeholders and collects feedback through several channels, including: the Therapeutic Goods Advertising Consultative Committee (TGACC); the TGA Consultative Committee consultation forum (TCC Forum); consultation processes for proposed regulatory changes; annual stakeholder surveys; and the TGA Contact Centre and Advertising Enquiries Portal.
Therapeutic Goods Advertising Consultative Committee
4.14 The TGACC was established in 2018. It represents stakeholders from consumer, health professional, media, industry and government bodies. The purpose of the TGACC is to: provide input to policies relating to the administration of the Therapeutic Goods Advertising Code; provide a forum for engagement on emerging issues with respect to therapeutic goods advertising; assist with reporting activities with respect to advertising compliance; and provide input on the development of education and compliance priorities to address non-compliance of advertising for particular categories of therapeutic goods. The TGACC met three times during 2021–22 and twice in the first six months of 2022–23.
TGA Consultative Committee consultation forum
4.15 The TCC forum has a broader remit than the TGACC and covers the regulatory spectrum for therapeutic goods, including for issues related to cost recovery.38 Its membership includes industry and non-industry bodies involved in the manufacture, use and consumption of therapeutic goods. The terms of reference for the TCC forum are to: comment on strategic priorities; provide input on policy development and program implementation; discuss issues impacting on industry, non-industry and consumer groups; and consider topics raised by members. The TCC forum met twice in 2021–22. The TGA Industry Forum, a sub-committee of the TCC forum that provides feedback on industry specific issues, met once in 2021–22.
Consultation processes for proposed regulatory changes
4.16 The department undertakes consultation to seek feedback from key stakeholders in relation to proposed regulatory changes. In 2022, the department sought feedback 29 times in relation to the regulation of therapeutic goods. For example, in late 2022 to early 2023, it consulted stakeholders on reforms to the regulation of nicotine vaping products (Case Study 3).
|
Case study 3. Consultation on regulation of Nicotine Vaping Products |
|
On 1 October 2021, the entry for nicotine in Schedule 4 to the Standard for the Uniform Scheduling of Medicines and Poisons (Poisons Standard) was amended to capture all nicotine vaping products such as nicotine e-cigarettes, nicotine pods and liquid nicotine as prescription-only medicines.a The focus of the Australian Government’s approach to nicotine vaping products is to prevent children and adolescents from using these products while enabling adults to have access to them under a doctor’s prescription for the purpose of smoking cessation. From November 2022 to January 2023, the department conducted a consultation process on potential further reforms to the regulation of nicotine vaping products in Australia. A consultation paper published on the department’s Consultation Hub website stated that evidence was emerging that the 2021 reforms were not meeting their intended objectives. The department received about 4000 submissions from a range of stakeholders, including: health professional bodies and associations; vaping manufacturers; importers; retailers; and the public. The department provided its analysis of submissions to the Minister for Health and Aged Care in January 2023. In May 2023, the Minister for Health and Aged Care announced stronger regulation of all e-cigarettes, including: stopping the importation of non-prescription vaping products; restricting flavours; requiring pharmaceutic-like packaging; and banning all single use disposable vapes. |
Note a: The Poisons Standard is a record of decisions on the classification of medicines and chemicals into Schedules. It is a legislative instrument that includes 10 schedules, including Schedule 4 on prescription only medicines.
Annual stakeholder surveys
4.17 The department conducts an annual survey of consumers, users of the TGA’s Business Services39 and health professionals. The 2022 survey found a high level of awareness of rules around advertising therapeutic goods in Australia among health professionals who advertise or arrange the advertising of therapeutic goods. The department has implemented improvements based on survey feedback. For example, feedback from the 2020 and 2021 surveys noted users often found it difficult to navigate the TGA website. In response, the department established a ‘Transformation Program’ in 2021, which included redeveloping the website. The new website was launched in August 2022.
TGA Contact Centre and Advertising Enquiries portal
4.18 The department has set up the TGA Contact Centre, which includes a telephone line and email inbox to help with enquiries about medicines, medical devices and other therapeutic goods. The TGA Contact Centre produces a quarterly and annual internal report, with statistics on the number of emails and telephone calls received and actioned through the Contact Centre.
4.19 Separate to the Contact Centre, the department also maintains an Advertising Enquiries portal. In its 2021–22 annual report on advertising compliance (see paragraph 2.37), the department stated it had received 494 advertising enquiries from consumers, industry and third party advertisers in 2021–22 through the portal.
Is the investigation framework fit-for-purpose?
The department’s investigation policies and procedures for the regulation of therapeutic goods are not mature and do not fully comply with the Australian Government Investigations Standards. Investigator qualifications are not sufficiently monitored, processes for declaring conflicts of interest and complaints handling are not fit-for-purpose, and a quality assurance process has not been established.
4.20 The AGIS outlines standards for the conduct of investigations including standards for: the arrangements that support investigations, such as investigation policies and procedures; and case management, such as how reports of alleged non-compliance are received, recorded, assessed and accepted for investigation. The AGIS 2011 defines an investigation as:
a process of seeking information relevant to an alleged, apparent or potential breach of the law, involving possible judicial proceedings. The primary purpose of an investigation is to gather admissible evidence for any subsequent action, whether under criminal, civil penalty, civil, disciplinary or administrative sanctions. Investigations can also result in prevention and/or disruption action. The term investigation can also include intelligence processes which directly support the gathering of admissible evidence.40
4.21 A case becomes an ‘investigation’ when it moves to a compliance or investigation team for a compliance response (or in some cases, when the triage team makes further inquiries or undertakes a compliance response).41
Investigation Policy
4.22 The AGIS 2011 states that an entity ‘is to have clear written policy in regard to its investigative function’.42 The Health Products Regulation Group has had several versions of its Investigation Policy; the current policy was finalised in March 2019. The 2019 Investigation Policy includes two of the four key elements outlined in the AGIS 2011 (Table 4.3).
Table 4.3: 2019 Investigation Policy alignment with AGISa
|
AGIS requirement for investigation policy |
Investigation Policy |
|
a statement regarding the agency’s objectives in carrying out its investigation functions and use of sanctions |
■ |
|
a clear definition of activities applicable to the agency to which the AGIS apply. This should include a description of compliance activities that are not generally considered investigations by the agency |
◆ |
|
a statement regarding the agency’s responsibility to manage matters that are considered minor or routine |
■ |
|
a statement regarding the agency’s responsibility to refer criminal matters to the Australian Federal Police (AFP). This should include consideration of joint agency investigation teams, where appropriate |
◆ |
Key: ◆ Met ▲ Partly met ■ Not met
Note a: The ANAO examined the Investigation Policy against the AGIS 2011, as that was the version of the AGIS in force at the time the department’s investigation policy was finalised (March 2019).
Source: ANAO analysis of departmental information and the Australian Government Investigations Standards 2011.
4.23 The AGIS was updated in October 2022, with the new version stating ‘entities should have a policy regarding their investigation function’ and that this policy should include:
- an outline of the entities’ remit in the context of types of investigations conducted;
- statements regarding the application of AGIS for particular types of investigations;
- statements regarding the assignment of AGIS qualifications for investigation areas; and
- statements regarding the entities’ responsibility to refer matters or investigations to law-enforcement entities or other relevant bodies.
4.24 In June 2023, the department advised the ANAO that it was in the process of updating the Investigation Policy to align with the October 2022 AGIS. 43
Investigation procedures
4.25 The ANAO examined whether the Health Products Regulation Group’s written investigation procedures for compliance investigations met the requirements for written procedures in the AGIS (Table 4.4). Investigation procedures had been developed, however: the majority of procedures were in draft; and the storage of procedures was inconsistent across different platforms.
Table 4.4: Investigation procedures compliance with AGIS
|
AGIS requirement for written investigation procedures |
Investigation procedures |
|
Liaison with the media in regard to investigations (AGIS 1.10) |
▲ a |
|
Initial evaluation and actioning of each matter (AGIS 2.2) |
◆ |
|
Investigation management (AGIS 3.1) |
▲ b |
|
Finalising the investigation (AGIS 3.6) |
▲ b |
|
Exhibit handling procedures (AGIS 4.5) |
▲ b |
Key: ◆ Met ▲ Partly met ■ Not met
Note a: Rated as ‘partly met’ because it relates specifically to advertising and not does not cover other compliance areas, such as import and supply.
Note b: Rated as ‘partly met’ because some guidance has been drafted, but it has not been finalised.
Source: ANAO analysis of departmental information and the Australian Government Investigations Standards 2011.
4.26 The department advised the ANAO that a surge in workload in 2021 and 2022 related to the COVID-19 pandemic and changes to the regulatory framework for nicotine vaping products diverted resources from business improvement initiatives, including the creation and revision of standard operating procedures. As part of the 2022–23 governance project, the department outlined the status of each standard operating procedure (related to compliance for unapproved therapeutic goods), noting which ones would need updating to align with the new AGIS 2022 (see paragraphs 2.18 to 2.20).
Recommendation no.2
4.27 The Department of Health and Aged Care:
- finalise its investigation procedures for the regulation of therapeutic goods and ensure these procedures align with requirements of the Australian Government Investigations Standard 2022; and
- establish an internal control for the regular review and update of these investigation procedures.
Department of Health and Aged Care response: Agreed.
4.28 The Department of Health and Aged Care is taking steps to ensure the Therapeutic Goods Administration’s compliance and investigation procedures align with the revised Australian Government Investigations Standard 2022 and are up to date and fit for purpose. A delivery plan has been developed to ensure timely creation and revision, as appropriate, of standard operating procedures (SOPs) for conducting investigations. This includes the creation of a new SOP on governance that outlines requirements for maintaining investigation procedures.
Investigator qualifications
4.29 The AGIS 2011 recommended the following minimum level of training or qualification for investigations staff: Certificate IV in Government (Investigation), or its equivalent; or Diploma of Government (Investigation), or equivalent. The AGIS 2022 strengthened the section on investigator qualifications, stating the following requirements.
- To preserve the ongoing Australian government capability for investigations, a vocational and educational training (VET) qualification must be obtained, unless another qualification or internal training is determined as equivalent.
- Entities must document the required VET accredited qualification/s (or equivalency) to conduct particular types of investigations and the timeframe in which investigators should obtain the qualification.
4.30 Of the 37 investigators who conducted investigations sampled by the ANAO, 73 per cent (27) had a minimum level of investigator qualification as outlined by the AGIS (Table 4.5). Of the 10 investigators who did not have qualifications, five were working to obtain a minimum level of qualification at the time of the audit and one was a junior officer who had been on rotation to the investigations team. The department does not maintain records of its investigators’ qualifications. When the ANAO requested evidence of investigator qualifications, the department manually confirmed qualifications with each investigator and prepared a list for the ANAO. Maintaining a register of investigator qualifications could help to ensure that investigators meet the AGIS requirement for the minimum level of qualification.
Table 4.5: Investigator qualifications for investigators in ANAO samples
|
ANAO sample |
Investigators with minimum level of qualification |
Investigators without qualifications |
Total |
|
Product and import compliance investigators |
14 (78 per cent) |
4a (22 per cent) |
18 |
|
Advertising compliance investigators |
10 (62.5 per cent) |
6b (37.5 per cent) |
16 |
|
Investigators for both product and import and advertising compliance |
3 (100 per cent) |
0 (0 per cent) |
3 |
|
Total |
27 (73 per cent) |
10 (27 per cent) |
37 |
Note a: Includes three investigators who were working to obtain a minimum level of qualification.
Note b: Includes two investigators who were working to obtain a minimum level of qualification.
Source: ANAO analysis of departmental information.
4.31 As noted in paragraph 3.12, triage officers sometimes took action on cases, such as issuing a warning or requesting the removal of an advertisement. As the AGIS definition of investigations includes prevention and disruption actions, this would suggest that some triage officers also require formal qualifications. The ANAO did not examine the investigation qualifications of triage officers.
Recommendation no.3
4.32 The Department of Health and Aged Care:
- ensure that investigators maintain a minimum level of investigator qualification, as required by the Australian Government Investigations Standard 2022; and
- keep appropriate records of investigator qualifications.
Department of Health and Aged Care response: Agreed.
4.33 The Department of Health and Aged Care is taking steps to ensure all investigators achieve the minimum qualifications of a Certificate IV in Government Investigations (or equivalent) as required by the Australian Government Investigation Standard. As noted in the report, a majority of investigators had obtained, or were working towards obtaining, the minimum level of investigator qualifications required. The Therapeutic Goods Administration Investigations Policy will be updated to require that investigators who do not possess appropriate qualifications must commit to obtaining the minimum qualifications within six months of their commencement. A qualifications register has been established and is being maintained.
Ethical conduct arrangements
4.34 The ‘Ethical Conduct’ section of the AGIS 2011 (section 1.9) states that agencies must:
- conduct investigations in accordance with the Australian Public Service (APS) Values, and APS Code of Conduct44; and
- have a procedure ensuring complaints about the conduct of investigations are handled in a timely, appropriate and comprehensive manner.45
Declarations of interest
4.35 The APS Code of Conduct, which is set out in section 13 of the Public Service Act 1999, requires that APS employees take reasonable steps to avoid any real or apparent conflict of interest. Where conflicts cannot be avoided, the APS Code of Conduct, section 29 of the Public Governance, Performance and Accountability Act (PGPA Act), and section 16 of the Public Governance, Performance and Accountability Act Rule 2014 (PGPA Rule) require that employees must disclose details of any material personal interest. The Australian Public Service Commission guide APS Values and Code of Conduct in Practice states that entities may choose to require written declarations of interest of employees at particular risk of conflict of interest, such as those involved in ‘regulating individual or business activities’.46
4.36 The department’s Accountable Authority Instructions state that: officials must disclose any material personal interest that relates to the affairs of the department or the Commonwealth, consistent with the PGPA Act section 29 and PGPA Rule section 16; and officials must document the disclosure, including any actions already taken to mitigate any real or perceived conflicts. The department’s Conflict of Interest Policy states employees undertaking investigations and other compliance activities must identify and effectively manage conflict of interest risks to ensure that ‘recommendations made by those employees are transparent, impartial and free of bias’.
4.37 The Regulatory Compliance Branch drafted an ‘Integrity Policy’ in 2021–22, which outlines additional requirements. The draft Integrity Policy outlined stronger requirements, with branch members to be asked to provide the branch head with an annual written statement that provides assurance that neither the staff member nor their immediate family hold financial or other interests in bodies for which the department has responsibility. The draft Integrity Policy, once finalised, will instruct staff to file these annual declarations in a specified folder on the department’s electronic management system. The ANAO found that the policy had not been finalised and the folder did not contain any declarations. In March 2023, the department confirmed that annual declarations referred to in the draft Integrity Policy had not been completed and further advised that only one declaration had been made in accordance with the departmental policy between 1 July 2021 and 31 December 2022. In June 2023, the department advised the ANAO that: the Integrity Policy was being progressed but had not been finalised; and officers in the Regulatory Compliance Branch would be required to complete declarations of interest going forward.
Recommendation no.4
4.38 The Department of Health and Aged Care:
- establish an internal control to ensure that officials involved in investigations and compliance activities make and manage declarations of interest; and
- keep appropriate records of declarations of interest.
Department of Health and Aged Care response: Agreed.
4.39 The Department of Health and Aged Care (the department) will review the specific requirements regarding the declaration and management of conflicts for employees performing investigation and compliance functions within the department. The required adjustments would be either documented separately or included in the department’s Conflict of Interest Policy. The department will also investigate improvements for recording and storage of records of declarations of interest. In addition, the department will finalise the implementation of the Integrity Policy for the Regulatory Compliance Branch.
Complaints handling procedures
4.40 The ‘Ethical Conduct’ section of the AGIS 2011 (section 1.9) states that agencies should have a procedure ensuring that complaints about the conduct of investigations are handled in a timely, appropriate and comprehensive manner. There is additional guidance for regulators on managing complaints.
- The Regulator Performance Guide outlines that regulators should have easy-to-access and transparent complaints and feedback handling procedures.47
- The Commonwealth Ombudsman’s Better Practice Guide to Complaint Handling (2009) outlines that a complaint handling system should be accessible to clients. Accessibility rests on two features — public awareness of the system and effective access options.48
- The updated Commonwealth Ombudsman’s Better Practice Complaint Handling Guide (2023) outlines key design principles for complaints handling systems, the first of which is that the system should be user-centred, simple to access and easy to use. This includes making complaints pages readily accessible from the home page of a website so that people can reach it in one or two clicks.49
4.41 The ANAO’s assessment of the department’s complaints handling procedures against this guidance is outlined at Table 4.6.
Table 4.6: Alignment with Commonwealth complaint handling guidance
|
Complaint handling guidance |
Alignment |
|
Complaints handling procedures established and transparent |
▲ |
|
Customer service standards for responding to complaints easily accessible on website (including standards for timeliness) |
◆ |
|
Complaints form easily accessible on website |
▲ |
|
End-to-end complaints management system established |
▲ |
|
Data on complaints and complaint handling timeliness collected and analysed |
▲ |
Key: ◆ Aligned ▲ Partly aligned ■ Not aligned
Source: ANAO analysis of departmental information.
4.42 The 2022 governance project identified that it was not clear from the website how a person would make a complaint. The governance project plan notes that a complaints procedure ‘requires preparation’.
4.43 The Commonwealth Ombudsman’s Better Practice Complaint Handling Guide states that complaints‘ should be recorded in an electronic system capable of producing complaint data’ and entities ‘should have an electronic system for end-to-end complaint management if it delivers services direct to the public’.50
4.44 The ANAO examined the department’s complaint handling processes and found the following.
- The department does not have an electronic system for end-to-end complaint management.
- The department does not maintain a single register of complaints. The department’s complaints management policy states that it has ‘a system of decentralisation where the management, monitoring and reporting of complaints is undertaken by individual business areas’. Complaints are also received and triaged through several entry points.
- The department advised the ANAO in April 2023 that it does not register all complaints relating to its regulation of therapeutic goods, although it maintains a list of complaints that had been escalated internally.51 The list included 29 entries for 2018–19 to 2021–22, with limited information about each complaint.
- There are ‘customer service standards’ on the TGA website relating to its regulation of therapeutic goods, which state that it will (among other things): ‘acknowledge letters and emails within five working days’. In response to an ANAO request for statistics on the percentage of responses that met the customer services standards in 2021–22, the department stated that ‘We are unable to advise what timeframe the TGA has replied to complaints/feedback over extended period as reporting is a manual process’.
4.45 A 2022 internal audit into complaints handling found that the department’s management of complaints needed improvement. The department agreed to the internal audit recommendations that it should:
- establish an overarching complaints management framework to enable appropriate governance and oversight of all complaints received by the department;
- update the structure and contents of the complaints management policy;
- establish appropriate communication and training to raise awareness of the current policy and ensure that it forms the basis of operational processes and procedures for respective line areas handling complaints; and
- establish a central reporting mechanism to enable better understanding and visibility of the types, volume, frequency and response timeframe to complaints received.
Recommendation no.5
4.46 The Department of Health and Aged Care establish:
- clear complaint handling channels that are accessible and easy to use; and
- a system for end-to-end complaint management and reporting.
Department of Health and Aged Care response: Agreed.
4.47 The Department of Health and Aged Care (the department) receives and triages complaints through several entry points including a form accessible via the ‘Contact us’ page on the department’s website. The department is investigating a ‘single front door’ interface to lodge all complaints on the department’s website, enabling the triage and distribution of complaints to the area responsible through an enterprise-wide client relationship management system. This would provide an improved process for lodging complaints and clearer complaint handling channels with centralised reporting capability.
Quality review processes
4.48 The AGIS 2011 sets out guidance on conducting quality reviews ‘to establish whether the investigation was conducted in a way that complied with AGIS’. The AGIS 2022 includes stronger requirements for quality review including entities having an investigations Quality Assurance Policy in place that includes quality assurance activities; and conducting one formal external quality assurance activity every two years. As of January 2023, the department did not have a quality assurance program for its investigations related to therapeutic goods.
Recommendation no.6
4.49 The Department of Health and Aged Care develop an Investigations Quality Assurance Policy, as required by the Australian Government Investigations Standard 2022.
Department of Health and Aged Care response: Agreed.
4.50 The Department of Health and Aged Care will finalise the investigations policy and procedures for the Regulatory Compliance Branch as recommended and develop an investigations quality assurance policy to further strengthen its governance arrangements. The quality assurance policy will outline the quality assurance activities (reviews and audits) required, including their type and frequency, to assess investigative performance.
Are compliance investigations and actions undertaken effectively?
The department’s compliance investigations and activities for the import and supply of unapproved therapeutic goods, and for the advertising of therapeutic goods, are consistent with the Australian Government Investigations Standards and are undertaken effectively, except for a lack of investigation planning for serious non-compliance cases and supervisor review.
4.51 The department conducts investigations and undertakes compliance responses related to offences under the Therapeutic Goods Act. There are three main investigation and compliance response teams within the Regulatory Compliance Branch:
- Product and Import Compliance (principally managing import and supply matters, but also a small number of manufacturing and export matters);
- Advertising and Product Investigations; and
- Product Investigations (managing investigations of serious non-compliance, see paragraph 3.11).52
4.52 The Therapeutic Goods Act outlines offences related to the import, export, manufacture or supply of therapeutic goods (Appendix 3) and provides the department with compliance and enforcement powers, ranging from enforceable undertakings and infringement notices to criminal and civil penalty proceedings.
4.53 The department may conduct non-statutory activities before using the compliance powers outlined in the Therapeutic Goods Act. Non-statutory activities include warning letters and informal engagement, including phone calls and emails. These activities serve an educative purpose, while also providing opportunities for the entity to voluntarily comply in advertising cases and for importers to provide evidence that imports are legal for import and supply cases.
4.54 In 2021–22, the department closed 8625 compliance cases in relation to the import, supply, export or manufacturing of unapproved therapeutic goods and 2376 cases related to the advertising of therapeutic goods.53 To determine whether the department had effectively undertaken investigations and compliance actions in 2021–22, the ANAO examined random samples of import and supply cases (excluding serious non-compliance cases) and advertising cases, as well as a targeted sample of serious non-compliance cases (Table 4.7).
Table 4.7: Cases closed in 2021–22 and ANAO samples
|
|
Number of cases closed |
||
|
|
Import, supply, export and manufacturing |
Advertising |
Serious non-compliance |
|
Population of cases closed |
8625a |
2376 |
49a |
|
ANAO sample of cases closed |
140 |
131 |
20b |
Note a: The import, supply, export and manufacturing population is taken from the department’s case management system for import, supply, export and manufacturing cases, which is also used by the team that investigates serious non-compliance. Serious non-compliance matters are primarily concerned with import and supply matters, although advertising non-compliance may also be considered. These cases were handled by the Product Investigations team.
Note b: Attributes targeted for the serious non-compliance sample included cases with an enforcement outcome; cases where more than 10,000 units of the product were destroyed; and cases relating to COVID-19, nicotine vaping products and sports supplements. The ANAO used this sample to examine elements of case management, such as investigation plans and infringement notices, that do not feature in routine investigations.
Source: ANAO analysis.
4.55 The ANAO examined the sampled cases against the AGIS 2011 for case management and the conduct of investigations (Table 4.8).
Table 4.8: Alignment with AGIS for investigation case management and conduct
|
AGIS requirement |
Alignment with the AGIS |
||
|
|
Import and supply |
Advertising |
Serious non-compliance |
|
Investigation plans prepared (AGIS 3.3) |
N/Aa |
N/Aa |
▲ b |
|
Key decisions and activities documented (AGIS 3.1 and 3.5) |
◆ |
◆ |
▲ c |
|
Critical decisions made by an appropriate officer (AGIS 3.5.5) |
◆ |
◆ |
◆ |
|
Supervisors review investigations at appropriate intervals to ensure adherence with the AGIS and investigation plans (AGIS 3.5.3) |
▲ d |
▲ e |
▲ f |
Key: ◆ Aligned ▲ Partly aligned ■ Not aligned
Note a: Investigation plans are not prepared for routine import and supply and advertising investigations as these generally involve a simple compliance response, such as issuing a warning letter and requesting the takedown of an online advertisement.
Note b: Planning documentation was on file for six of the 20 investigations of serious non-compliance examined (see paragraph 4.59).
Note c: Records were not complete for four of 20 serious investigations examined (see paragraph 4.70).
Note d: Supervisor review was not evident for two of 117 cases where supervisor review was required (see paragraph 4.76).
Note e: Of 131 advertising cases, 18 did not have appropriate records and 12 did not have evidence of appropriate supervisor review (see paragraph 4.77).
Note f: Supervisor review did not include ensuring adherence with the AGIS or investigation plans (see paragraph 4.78).
Source: ANAO analysis of departmental information.
Investigation plans prepared
4.56 Section 3.3 of the AGIS 2011 states that ‘each investigation should commence with an overall planning process and where possible result in a written investigation plan. This plan should be referred to and updated during the investigation’. The AGIS lists further requirements for the planning process, including that it should identify and manage risks, setting out that ‘agencies should ensure risk management is incorporated in decision making throughout an investigation’ and that this is particularly important in areas such as investigation planning.
Routine investigations
4.57 Investigation planning and operational risk management documentation is generally not undertaken for routine investigations. The department has a standard investigation plan template for investigations related to therapeutic goods, but it does not require the completion of investigation plans for routine investigations and compliance responses.
4.58 In 2021–22, the department processed some COVID-19 related cases as part of a bulk round (discussed at paragraphs 3.17–3.18). Planning for this bulk activity was documented in a proposal that included detail on the issues that needed to be addressed, the steps to be taken and operational risks. Of the 140 cases examined by the ANAO, 22 were processed as part of the ‘Phase 1 bulk mailout pilot’ for a range of COVID-19 related products.
Investigations of serious non-compliance
4.59 The department completed planning documentation for six of 20 investigations into serious non-compliance examined by the ANAO. Of the six investigations with planning documentation, none used the investigation plan template. The planning documents included initial assessment reports, situation reports and investigation plans. None of these planning documents fully complied with AGIS requirements. While all plans outlined the conduct or breach to be investigated and included background detail on the case, not all plans outlined the objective and scope of the investigation, and none outlined the investigation team structure or how risks would be managed.
4.60 Under the AGIS, supervisors should review investigations at appropriate intervals to ensure adherence with the AGIS and investigation plans. In the absence of investigation plans, supervisors are not well placed to monitor the performance of investigations or to ensure that risks are being appropriately managed. The documentation of investigation plans also helps to ensure continuity, particularly in circumstances where an investigator is unable to complete an investigation.
|
Opportunity for improvement |
|
4.61 The Department of Health and Aged Care could prepare investigation plans, including risk assessments, for investigations of serious non-compliance related to the regulation of therapeutic goods. |
Key decisions and activities documented
4.62 The department’s workflow for product and import and advertising case management, including key decision points and activities, is outlined in Appendix 4.
Product and import activities
4.63 In 2021–22, the department undertook 8988 actions relating to import, supply, manufacture and export across 8625 cases (Table 4.9). These actions included case closure where the department determined that there had been no offence and the referral of cases where the department determined the matter to be outside of its jurisdiction. This determination could be made at the triage point by an assessment officer or by an officer in one of the compliance response and investigation teams.
Table 4.9: Import, supply, manufacturing and export compliance actions for cases closed in 2021–22
|
Compliance action |
2021–22 |
|
Warning letter issueda |
8015 |
|
No breach or offence identified |
357 |
|
Goods released under Personal Importation Scheme |
285 |
|
Referred to an external agency |
227 |
|
Infringement notice issued |
74 |
|
Referred internally |
27 |
|
Criminal prosecution |
2 |
|
Referred to the Commonwealth Director of Public Prosecutions |
1 |
|
Totalb |
8988 |
|
Units of goods referred to the Australian Border Force for destructionc |
5,137,491 |
Note a: The category ‘warning issued’ includes goods destroyed as prohibited imports and goods re-exported.
Note b: There can be multiple actions per case resulting in a greater number of actions than the total number of cases closed in the period.
Note c: Units refers to single dosage unit (for example, one tablet, one tub of powder or one device).
Source: ANAO representation of data in the Therapeutic Goods Administration Performance Report 2021–22.
4.64 The most common compliance action taken for import and supply cases in 2021–22 was ‘warning letter issued’. After issuing a warning letter to importers, the department may take one or more of the following actions.
- Write to the Comptroller-General of Customs.54 Correspondence may include a section 56A certificate, notifying Australian Border Force (ABF), within the Department of Home Affairs, that exemptions or approvals were not in place when an importer attempted to import goods. This facilitates destruction of the goods by the ABF under the Customs Act 1901.
- Release the goods to the importer. Reasons for releasing goods include: the importer has provided sufficient evidence for goods to be released under the Personal Importation Scheme55; the type and quantity of goods is determined to be low risk and can be released under the Personal Importation Scheme without evidence; or the import is lawful under the Special Access Scheme.56
- Issue an infringement notice to the importer or supplier in accordance with Part 5A-2 of the Therapeutic Goods Act.
- Conduct a further investigation.
- Take no further action.
4.65 The final action the department recorded for the 140 import and supply cases57 and the 20 serious non-compliance cases examined by the ANAO are presented in Table 4.10.
Table 4.10: Import and supply and serious non-compliance case final actions, cases closed in 2021–22 (ANAO samples)
|
Final action type |
Import and supply |
Serious non-compliance |
|
Letter issued to the Comptroller-General to facilitate destruction of the goods by the ABF (following a warning letter to the importer) |
117 |
8 |
|
Infringement notice issued |
– |
4a |
|
Goods recommended to be released to the importer |
18 |
2 |
|
Case referred internally for further investigation |
2 |
– |
|
Goods seized and destroyed by the department |
– |
1 |
|
Other outcome |
3 |
5b |
|
Total |
140 |
20 |
Note a: The department received confirmation that the notices had been paid in three cases and it withdrew the infringement notice for one case.
Note b: This includes four ‘no further action’ cases.
Source: ANAO analysis.
Advertising activities
4.66 The department reported that it had recorded 1973 advertising compliance actions in 2021–22 (Table 4.11). It is unclear from departmental reporting how many cases were associated with these actions. Actions included case closure where the department determined that there had been no breach and the referral of cases to other agencies where the department determined the case to be out of its jurisdiction.
Table 4.11: Advertising compliance actions recorded in 2021–22
|
Compliance action |
2021–22 |
|
Advertisements removed from digital platforms |
1174 |
|
Warning issueda |
343 |
|
No breach or offence identified |
334 |
|
Infringement notice issued |
110 |
|
Referred to an external agency |
6 |
|
Civil matters |
4 |
|
Criminal matters |
2 |
|
Totalb |
1973 |
Note a: The term ‘warning issued’ relates to all correspondence advising advertisers of an alleged breach of the legislation. This includes warning letters, emails and phone calls.
Note b: Total actions taken is not the same as total cases. There can be multiple actions per case (for example, a warning letter could be issued, and then multiple infringement notices could be issued for the same case) and one action (such as a warning letter) can be in response to multiple cases This figure also relates to the number of actions recorded in the financial year, not the number of actions taken for the cases closed during the year.
Source: ANAO representation of data presented in the Therapeutic Goods Advertising Compliance Annual Report 2021–22.
4.67 The final actions the department recorded for the 131 advertising cases examined by the ANAO are presented at Table 4.12. Of these, 75 per cent (98) of cases were closed with no further action. A compliance action was taken in 25 per cent (33) of cases — primarily warnings and requests for advertisements to be removed. In 10 of 33 cases where a compliance action was taken, this was done by a triage officer.
Table 4.12: Advertising case final actions, cases closed in 2021–22 (ANAO sample)
|
Final action type |
Number of cases |
|
Case closed — by triage officer |
75 |
|
Case closed — no record of who closed the case |
18 |
|
Case closed — by investigatora |
5 |
|
Subtotal — Cases closed with no compliance action takenb |
98 |
|
Warning issued and/or advertisements requested to be taken downc |
32 |
|
Infringement notice issued |
1 |
|
Subtotal — Cases closed after compliance action |
33 |
|
Total |
131 |
Note a: Cases were closed without compliance action because: two were linked to an existing investigation; one was referred internally; there was insufficient information to pursue one case; and no breach was identified in one case.
Note b: Cases were closed without compliance action for several reasons, including: no breach was identified at initial assessment; the advertisement was determined to be out of jurisdiction; the contravention was not a compliance priority; the case was linked to an ongoing investigation; the report had insufficient information; or contraventions could not be validated.
Note c: ‘Warning issued’ includes phone calls, emails and warning letters to advertisers. This includes 10 cases where the compliance response was taken by a triage officer.
Source: ANAO analysis.
4.68 One of the 131 advertising cases examined involved the use of enforcement powers under the Therapeutic Goods Act. In this case, the department issued six infringement notices to a pharmacy website that had been advertising nicotine vaping products (see Case Study 4).
|
Case study 4. Infringement notice case study |
|
On 10 February 2022, a high priority advertising case was created for an online pharmacy that was advertising nicotine vaping products and prescriptions for nicotine vaping products. The advertising to consumers of prescription only therapeutic goods that contain nicotine is generally prohibited in Australia.a The department wrote to the pharmacy on 11 and 14 February 2022 regarding the unlawful advertising of nicotine vaping products on two websites. The department sent two letters warning the advertiser that its online advertising was in contravention of the Therapeutic Goods Act. These letters were followed by phone calls between the advertiser and the department to discuss remediation. On 15 February, the advertiser wrote to the department outlining the remediation steps it had taken, which included taking the advertising down. On 22 February 2022, the department case manager prepared a brief for the delegate recommending six infringement notices be issued, three to the company and three to the Director of the company. The three notices to the company totalled $39,960, and the three notices to the company owner totalled $7992. The department noted in the brief that it was difficult to accept that the advertiser, a pharmacist, and his company were not aware that it was illegal to directly advertise prescription-only products to the Australian public. The brief also noted that the department had been taking strong enforcement action in relation to the unlawful advertising of nicotine vaping products. On 23 February 2022, the department issued six infringement notices for the unlawful advertising of nicotine vaping products. All six infringement notices were issued on the basis the owner and company contravened subsection 42DLB(1) of the Therapeutic Goods Act, where subsection (7) applied. All six infringement notices were paid. |
Note a: The Therapeutic Goods Act outlines offences and penalties for advertising prescription only therapeutic goods that contain nicotine to the public: subsections 42DLB(1) and 42DLB(7) outline criminal offences; and subsections 42DLB(1) and 42DLB(7) outline civil penalties.
4.69 For the import, supply and advertising cases closed in 2021–22, the department issued 184 infringement notices totalling about $1.8 million.
Documentation of key decisions and activities
4.70 Section 3.5.1 of the AGIS 2011 states that activity during investigations should be recorded electronically or in written form on a suitable investigations management system. The department has two investigation management systems that are used for case management — one for product and import cases and one for advertising cases (see paragraph 3.8 for further discussion of the case management systems). To determine whether the management of cases meets AGIS requirements and whether key decisions and activities had been documented, the ANAO examined case records on the case management systems and electronic document management system.
- Import and supply: The ANAO examined the case records for evidence that the following standard activities occurred and were appropriately documented: case creation; issue of documents such as warning letters and section 56A certificates; and case closure.58 The ANAO found that the department had appropriately documented key decisions and activities for all 140 cases examined.
- Advertising: The ANAO examined the case records for evidence that the following standard activities occurred: case creation; preliminary assessment; issue of warning letters or other compliance activities; and case closure. The ANAO found that the department had documented key activities for 130 of the 131 cases examined. For one case, the department had not appropriately documented why the case was closed without proceeding to investigation. However, for 18 cases, the identity of the decision-maker was not clear (discussed at paragraph 4.77). The outcome descriptions in the case management system were vague (as discussed at paragraph 3.13).
- Investigations of serious non-compliance: The ANAO examined the case records for evidence of the same standard activities as the import and supply sample, with the addition of infringement notices and evidence briefs. Key decisions and activities were appropriately documented for 16 of the 20 cases examined. Records were not complete for four of the 20 cases examined, including one case where a section 56A certificate was issued and one case where an infringement notice was issued.59 While a clear timeline of events was not evident for the two cases, evidence relating to the approval of the relevant documents was on file.
Critical decisions made by an appropriate officer
4.71 Section 3.5.5 of the AGIS states that ‘All critical decisions made during an investigation should be made by an appropriate officer and documented on the investigation file or electronic system.’
4.72 Some actions or decisions must be completed by officers who have been delegated the appropriate authority by the Secretary of the department under the Therapeutic Goods Act. These actions include: recommending that the ABF seize and destroy goods through a section 56A certificate; and issuing infringement notices.60 For all import and supply, advertising and serious non-compliance cases examined by the ANAO, all decisions that were made under a delegated authority were made by officers with an appropriate delegation. These decisions were appropriately documented on the case files (see paragraph 4.70).
4.73 For cases of serious non-compliance, cases are sometimes referred to the department’s Enforcement Committee for advice. The role of the Enforcement Committee is to consider cases and make (non-binding) recommendations to the delegate on whether proposed enforcement action is appropriate and proportionate. Two cases in the ANAO’s serious investigation sample were considered by the Enforcement Committee.
Supervisor review
4.74 Section 3.5.3 of the AGIS states that ‘supervisors should review investigations at appropriate intervals to ensure adherence with the AGIS and investigation plans.’
4.75 Of the 20 cases of serious non-compliance examined by the ANAO:
- seven did not involve a compliance response — the department’s procedure did not require supervisor review for these cases; and
- 13 cases involved a compliance response — for all of these cases appropriate supervisor review was evident.
4.76 Of the 140 product and import cases examined by the ANAO:
- 23 cases did not involve a compliance response — the department’s procedure did not require supervisor review for these cases61; and
- 117 cases involved a compliance response — for these cases, appropriate supervisor review was evident for 115 of 117 cases.
4.77 Of the 131 advertising cases examined by the ANAO:
- 51 cases did not require supervisor review until after case closure, according to the department’s business practices62;
- 80 cases should have had supervisor review. Of these:
- 50 (62.5 per cent) had evidence of appropriate supervisor review on file. Supervisor review involved: the supervisor corresponding with case officers over compliance actions taken or case closures; and supervisors finalising files that did not proceed to further investigation after assessment.
- 18 cases (22.5 per cent) did not include the name of the supervisor or the name of the case officer in the case records and the ANAO could not test whether supervisor review had taken place; and
- 12 cases (15 per cent) did not have evidence of appropriate supervisor review in the case records.
4.78 For the cases examined that did have evidence of supervisor review, this review did not include ensuring adherence with the AGIS or adherence with investigation plans (as investigation plans were largely not prepared, which is discussed at paragraphs 4.57–4.61).
|
Opportunity for improvement |
|
4.79 The Department of Health and Aged Care could improve processes and control to ensure that supervisors are reviewing the work of officers involved in compliance activity related to therapeutic goods at appropriate intervals and verifying adherence to the Australian Government Investigations Standard 2022 and, where relevant, investigation plans. |
Appendices
Appendix 1 Entity response

Appendix 2 Improvements observed by the ANAO
1. The existence of independent external audit, and the accompanying potential for scrutiny improves performance. Improvements in administrative and management practices usually occur: in anticipation of ANAO audit activity; during an audit engagement; as interim findings are made; and/or after the audit has been completed and formal findings are communicated.
2. The Joint Committee of Public Accounts and Audit (JCPAA) has encouraged the ANAO to consider ways in which the ANAO could capture and describe some of these impacts. The ANAO’s 2023–24 Corporate Plan states that the ANAO’ s annual performance statements will provide a narrative that will consider, among other matters, analysis of key improvements made by entities during an ANAO audit process based on information included in tabled performance audit reports.
3. Performance audits involve close engagement between the ANAO and the audited entity as well as other stakeholders involved in the program or activity being audited. Throughout the audit engagement, the ANAO outlines to the entity the preliminary audit findings, conclusions and potential audit recommendations. This ensures that final recommendations are appropriately targeted and encourages entities to take early remedial action on any identified matters during the course of an audit. Remedial actions entities may take during the audit include:
- strengthening governance arrangements;
- introducing or revising policies, strategies, guidelines or administrative processes; and
- initiating reviews or investigations.
4. In this context, the below actions were observed by the ANAO during the course of the audit. It is not clear whether these actions and/or the timing of these actions were planned in response to proposed or actual audit activity. The ANAO has not sought to obtain assurance over the source of these actions or whether they have been appropriately implemented.
Table A.1: Improvements observed by the ANAO
|
Actions observed during the course of the audit |
Report paragraphs |
|
As part of the 2022–23 governance project, the department has: completed a stocktake of standard operating procedures, templates and policies; reviewed the new Australian Government Investigations Standard (AGIS) 2022 to determine updates needed; updated one standard operating procedure; and prepared an Advertising and Product Investigations Induction Manual for new starters. |
2.19 |
|
The department released an updated Education Plan in February 2023. The 2023 Education Plan outlined education activities that aligned with each of the 2022–23 import, advertising and supply compliance priorities. The 2023 plan also identified activities relating to regulatory changes and emerging issues, such as medical device reforms and information for advertisers of therapeutic goods. |
4.9 |
Appendix 3 Offences and civil penalties related to the import, export, manufacture, supply and advertising of therapeutic goods, Therapeutic Goods Act 1989
Table A.2: Offences under the Therapeutic Goods Act 1989 — Import, export, manufacture or supply
|
Offence or penalty type |
Section and subsection of the Therapeutic Goods Act 1989 |
|
Import, export, manufacture or supply of therapeutic goods including prescription medicines |
19B and 19D |
|
Import, export or supply of goods that do not comply with standards |
14 and 14A |
|
Import, export, manufacture or supply of counterfeit goods |
42E and 42EA |
|
Import, export, manufacture or supply of biologicals |
32BA–BD and 32BF |
|
Import, export or supply of medical devices |
41MI and 41MIB |
Source: ANAO analysis of departmental information and the Therapeutic Goods Act 1989.
Table A.3: Offences and civil penalties under the Therapeutic Goods Act 1989 — Advertising
|
Offence or penalty type |
Subsection of the Therapeutic Goods Act 1989 |
|
Advertisement of unapproved therapeutic goods |
42DL(1), (2) and (3) |
|
Advertisement that includes prohibited representationsa |
42DL(5) and 42DLB(2) |
|
Advertisement that includes restricted representationsb |
42DL(7) and 42DLB(4) |
|
Advertisement of prescription medicines (including therapeutic goods that appear on schedule 3, 4 or 8 of the Standard for the Uniform Scheduling of Medicines and Poisons (the Poisons Standard)c |
42DL(10) and 42DLB(7) |
|
Non-compliance with the Therapeutic Goods Advertising Code |
42DM and 42 DMA |
Note a: Prohibited representations (under Schedule 2 of the Therapeutic Goods Act 1989) include representations that refer to: neoplastic diseases (all types of cancer); sexually transmitted diseases; HIV/AIDs; Hepatitis C virus; mental illness; and abortifacient action.
Note b: Restricted representations are representations that refer to a form of a disease, condition, ailment or defect that is a serious form within the meaning of s28 of the Advertising Code.
Note c: The Poisons Standard is a record of decisions on the classification of medicines and chemicals into schedules. It is a legislative instrument that includes 10 schedules, including Schedule 3 – Pharmacist only medicines, Schedule 4 – Prescription only medicines and prescription animal remedies, and Schedule 8 – controlled drugs.
Source: ANAO analysis of departmental information and the Therapeutic Goods Act 1989.
Appendix 4 Case management workflow
Figure A.1: Product and import and advertising case management workflow
Note a: Investigations of serious non-compliance may commence following second-pass assessment or if compliance has not been achieved after initial compliance action.
Source: ANAO analysis of departmental information.
Footnotes
1 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 3.
2 Department of Health and Aged Care, Annual Report 2021–22, p. 26.
3 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 3.
4 Department of Health and Aged Care, Annual Report 2021–22, p. 26.
5 The department maintains a website for the TGA (tga.gov.au) that is separate from the main departmental website (health.gov.au). The Regulatory Practice and Support division also includes the Office of Drug Control.
6 The Therapeutic Goods Act 1989 exempts certain products from the requirement to be entered on the ARTG as well as provides pathways for unapproved therapeutic goods to be lawfully imported, manufactured and supplied in Australia through the Personal Importation Scheme, Special Access Scheme, the Authorised Prescriber Scheme and for clinical trials and other purposes.
7 The Therapeutic Goods Act 1989 exempts certain products from the requirement to be entered on the ARTG as well as provides pathways for unapproved therapeutic goods to be lawfully imported, manufactured and supplied in Australia through the Personal Importation Scheme, Special Access Scheme, the Authorised Prescriber Scheme and for clinical trials and other purposes.
8 Annual charges for inclusion on the ARTG are outlined in the Therapeutic Goods (Charges) Regulations 2018.
9 Auditor-General Report No. 38 2018–19 Application of cost recovery principles, paragraph 7.
10 Auditor-General Report No. 30 2013–14 Administering the Code of Good Manufacturing Practice for Prescription Medicines, paragraph 9.
11 Auditor-General Report No. 3 2011–12 Therapeutic Goods Regulation: Complementary Medicines, paragraph 18.
12 The Australian Government Investigations Standards (2011) were replaced by the Australian Government Investigations Standard (2022) in October 2022.
13 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 8. Responsibility for the Regulator Performance Guide and deregulation policy coordination was moved to the Department of Finance on 1 July 2022. In December 2022, the Department of Finance revised and republished the guide as the RMG 128 Regulator Performance. There was no change to reporting requirements.
14 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 7.
15 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 9.
16 The Health Products Regulation Group is part of the Department of Health and Aged Care and comprises the Therapeutic Goods Administration and the Office of Drug Control. The Health Products Regulation Group is responsible for the regulation of medicines, medical devices, biologicals, and controlled drugs.
17 The Australian Government Investigations Standards (2011) were replaced by the Australian Government Investigations Standard (2022) in October 2022.
18 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 7.
19 The TGA Industry Forum is a sub-committee of the TGA Consultative Committee consultation forum, which is comprised of industry and non-industry bodies involved in the manufacture, use and consumption of therapeutic goods.
20 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 4. Responsibility for the Regulator Performance Guide and deregulation policy coordination was moved to the Department of Finance on 1 July 2022. In December 2022, the Department of Finance revised and republished the guide as the RMG 128 Regulator Performance. There was no change to reporting requirements.
21 Between 2015–16 and 2020–21, the department chose to publish an annual performance statistics report (which was not required) in addition to the self-assessment report (which was required).
22 Sections 37 to 40 of the PGPA Act, and sections 16E to 16F of the PGPA Rule.
23 Subsection 16E(2) item 5 of the PGPA Rule.
24 Section 16EA of the PGPA Rule.
25 Australian Government, Australian Government Investigations Standards, 2011, Chapter 2.
26 Australian Government, Australian Government Investigations Standards, 2011, section 2.1, p. 5.
27 Australian Border Force (the ABF) is Australia’s frontline border law enforcement and international customs service.
28 Australian Government, Australian Government Investigations Standards 2011, section 2.1. p. 5. The AGIS 2022 state that ‘entities should have an electronic investigation management system to record, collate and manage investigations’ (section 2.3).
29 The import and supply case management system also captures the cases involving the manufacture or export of unapproved therapeutic goods, of which there are few. For example, in 2021–22, of 9509 reports of non-compliance, three related to export and four related to manufacturing.
30 The department considers serious non-compliance to be ‘behaviour where an entity has clearly been advised on specific therapeutic goods legislation and knowingly takes actions that are in direct breach of this advice’. Wilful or knowing misconduct is conduct where an entity does not change their behaviour after being clearly notified that the behaviour is non-compliant. For example, an entity that has received a warning letter regarding the illegal importation of unregistered medicines, continues to illegally import unregistered medicines after receipt of the advice and does not take timely actions to become compliant, is displaying wilful or knowing misconduct.
31 After sending a warning letter, the department authorises the destruction of goods through a letter to the Comptroller-General of Customs unless the importer provides sufficient evidence for the goods to be released.
32 The department’s education activities are discussed in more detail in Chapter 4.
33 Department of Health and Aged Care, Therapeutic Goods Advertising Compliance Annual Report 2021–22, p. 19.
34 These advertisements and pieces of digital content related to goods such as nicotine vaping products, weight loss medication, medical devices and tanning products.
35 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 9. Responsibility for the Regulator Performance Guide and Deregulation Policy Coordination was moved to the Department of Finance on 1 July 2022. In December 2022, the Department of Finance revised and republished the guide as the RMG 128 Regulator Performance.
36 The five 2021–22 advertising compliance priorities listed in the Advertising Education Plan were: Therapeutic goods associated with COVID-19; Stem cell products; Medicinal cannabis; Performance and image enhancers; and Therapeutic goods used in the cosmetic and beauty industry.
37 Therapeutic Goods Administration, Compliance actions and outcomes [Internet], TGA, 2022, available from https://www.tga.gov.au/how-we-regulate/compliance-and-enforcement-hub/compliance-actions-and-outcomes [accessed 19 May 2023].
38 Cost recovery involves the Australian Government charging the non-government sector some or all of the costs of a specific government activity.
39 TGA Business Services is an online system used for conducting transactions with the department, including lodging applications for entry of products into the Australian Register of Therapeutic Goods and making payments.
40 Australian Government, Australian Government Investigations Standards, 2011, p. 1. An updated version of the AGIS was released in October 2022.
41 As discussed in Chapter 3, a case is created in one of the department’s two case management systems for each report of alleged non-compliance.
42 Australian Government Investigations Standards, 2011, p. 1. An updated version of the AGIS was released in October 2022.
43 The Australian Federal Police (AFP) released a new version of the AGIS in October 2022. The AFP web site states that the ‘AGIS does not have a date whereby implementation must be completed. Rather entities should start a proactive implementation plan and prioritise actions/requirements to meet the standard where possible in a reasonable timeframe’.
44 The AGIS 2022 states: ‘Entities and investigators must conduct investigations in accordance with the following: relevant statutory entity or independent entity Values, Code of Conduct, Code of Practice and/or Code of Ethics; and/or APS Values, Employment Principles and Code of Conduct in accordance with Australian Government legislation’.
45 The updated AGIS 2022 (section 1.2.2) similarly requires: ‘Entities must have procedures in place, relevant to legislation, which appropriately deal with complaints about the handling of investigations and cooperation with independent government oversight authorities investigating complaints made about an entity’s investigation.’
46 Australian Public Service Commission, APS Values and Code of Conduct in practice [Internet], APSC, 2018, paragraphs 5.2.7–5.2.12, available from https://www.apsc.gov.au/publication/aps-values-and-code-conduct-practice/section-5-conflict-interest [accessed 19 May 2023].
47 Department of the Prime Minister and Cabinet, Regulator Performance Guide, 2021, p. 7.
48 Commonwealth Ombudsman, Better Practice Guide to Complaint Handling, 2009, p. 11.
49 Commonwealth Ombudsman, Better Practice Complaint Handling Guide, 2023, pp. 10–12.
50 Commonwealth Ombudsman, Better Practice Complaint Handling Guide, 2023, pp. 16–17.
51 The department does not have protocols or criteria for this escalation process.
52 Investigations of serious non-compliance may commence following second-pass assessment if the alleged breach involves: repeated actions of non-compliance; wilful or knowing misconduct; or if compliance has not been achieved after initial compliance action.
53 This includes cases closed at triage and cases closed after investigation.
54 The ABF Commissioner also serves as the Comptroller-General of Customs.
55 Under the Personal Importation Scheme, individuals may import a three-month supply of some unapproved therapeutic goods for personal use or use by a person in their immediate family. See: Therapeutic Goods Administration, Personal Importation Scheme [Internet], TGA, available from https://www.tga.gov.au/products/unapproved-therapeutic-goods/personal-importation-scheme [accessed 21 April 2023].
56 Under the Special Access Scheme, health practitioners may prescribe unapproved therapeutic goods or sponsors may supply unapproved therapeutic goods if conditions are met. See: Therapeutic Goods Administration, Special Access Scheme (SAS) and Authorised Prescriber (AP) [Internet], TGA, available from https://www.tga.gov.au/products/unapproved-therapeutic-goods/special-access-scheme-sas-and-authorised-prescriber-ap [accessed 21 April 2023].
57 Excludes serious non-compliance cases, which are examined separately.
58 A section 56A certificate notifies the Australian Border Force (ABF) that exemptions or approvals were not in place when an importer attempted to import goods, which facilitates the destruction of the goods by the ABF under the Customs Act 1901; For cases that did not result in compliance action, such as when the department recommended that goods be released, the ANAO also examined whether this occurred.
59 A section 56A certificate notifies the ABF that exemptions or approvals were not in place when an importer attempted to import goods, and facilitates the destruction of the goods by the ABF under the Customs Act 1901.
60 Section 56A of the Therapeutic Goods Act allows a delegate to certify in writing that exemptions were not in place for specified periods, or that they did not consent to an import of a good. A section 56A certificate lists the importer’s details, the relevant good, a certification under specific sections of the Therapeutic Goods Act, and the details of the delegate.
61 The outcomes for these cases were: goods recommended to be released to the importer; case referred internally for further investigation; and no further action. The department internal policy does not require that investigations be reviewed if a case officer decides to recommend to the ABF that an import can be released.
62 This included cases where: the relevant case officer was a manager and could finalise the case themselves; the cases were closed because they did not pass the initial triage criteria; or the triage officer completed a digital platform removal request and then closed the case — with a supervisor to later review the closed case and ‘finalise’ the case in the case management system.